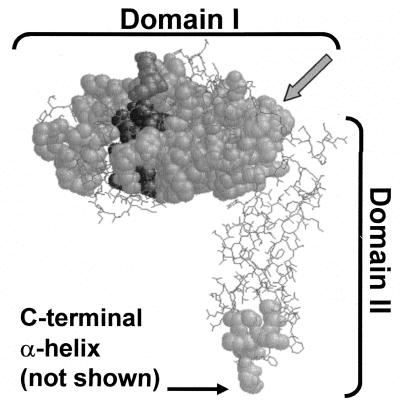
FIG. 1.
Positions of highly conserved sequences among PBP 5 homologues. The most highly conserved sequences of nine dd-carboxypeptidase PBPs were mapped onto the crystal structure of PBP 5 (4). Conserved segments are shown as space-filled residues superimposed onto nonconserved segments, which are represented in wireframe. Darker residues are in or near the active site. The carboxy-terminal α-helix was absent from the crystal structure, but its point of attachment to the base of domain II is indicated. The gray arrow indicates the residues in the α10 helix that form the boundary between domains I and II. Sequences used in the alignments are listed in the legend to Fig. 5.