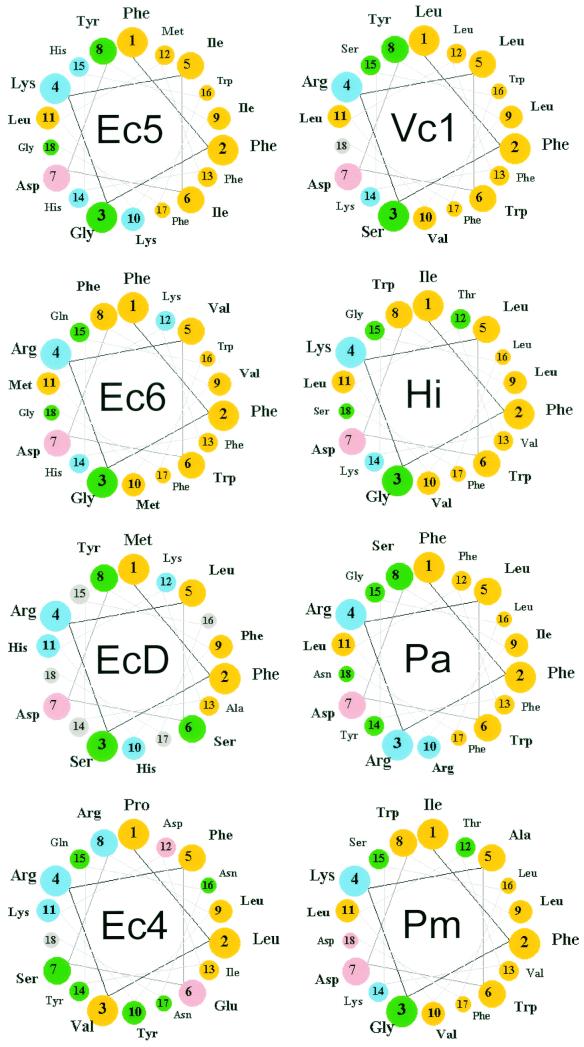
FIG. 4.
Helical wheel representations of the carboxy-terminal amino acid sequences of PBP 5 homologues. The carboxy-terminal α-helices of several dd-carboxypeptidase PBPs were determined by alignment with the sequence of PBP 5 (Fig. 5) and represented in a helical-wheel format. The first residue of the membrane anchoring sequence was defined as the amino acid immediately following the PEGN residues that mark the end of the crystal structure of PBP 5 in E. coli (4). Color key: yellow, nonpolar; green, polar uncharged; pink, acidic; blue, basic. Abbreviations (with accession numbers, when applicable): Ec5, E. coli PBP 5; Ec6, E. coli PBP 6; EcD, E. coli DacD; Ec4, E. coli PBP 4; Vc1, Vibrio cholerae group O1 strain N16961 (VC0947); Hi, Haemophilus influenzae PBP 5 (HI0029); Pa, Pseudomonas aeruginosa (PA3999); Pm, Pasteurella multocida DacA (PM1927).