Abstract
1. Fluid transport rate and oxygen consumption (QO2) were studied in rabbit gall-bladder preparations in vitro exposed on both sides to identical Ringer solutions with NaCl concentrations (and osmolarities) varying from 70 to 140 m-equiv Na+/l.).
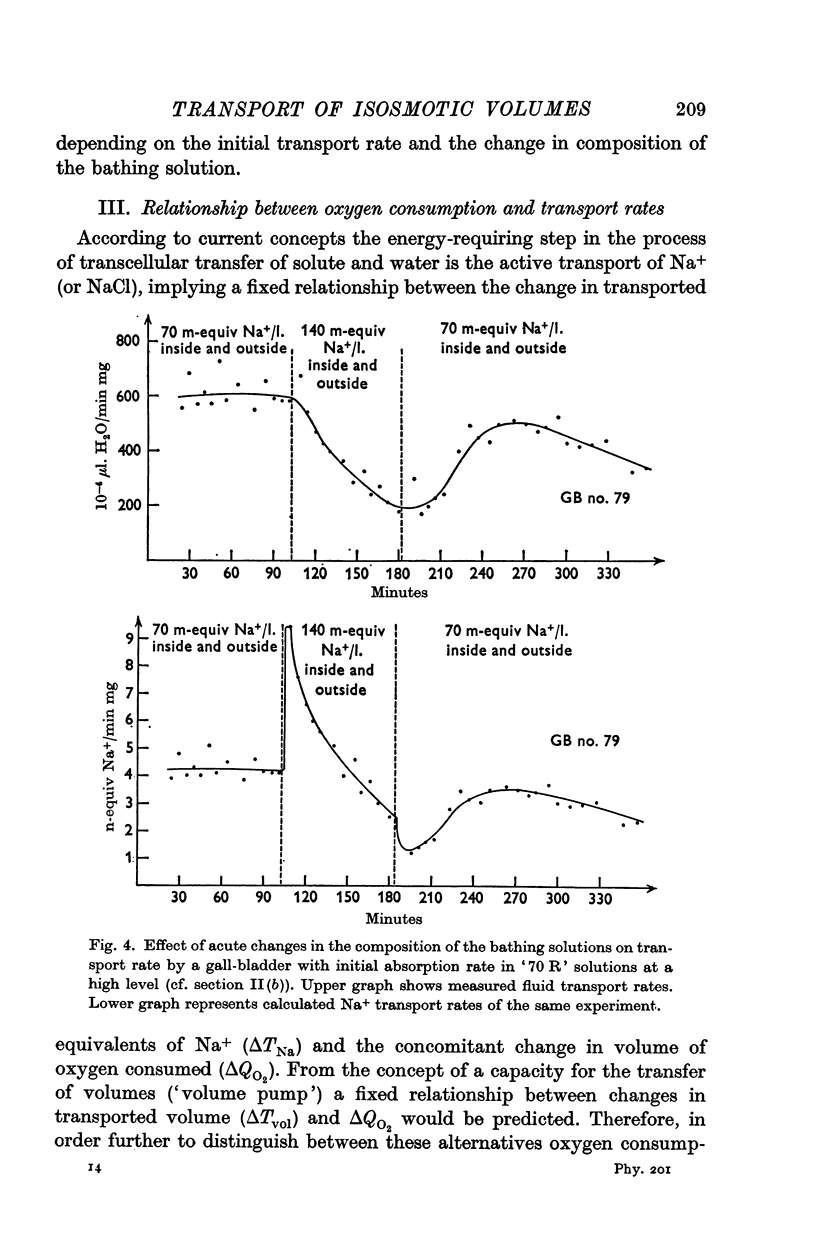
2. The time sequence of acute effects on transport rate resulting from sudden changes in the NaCl concentration of the bathing solutions indicated that, (a) as a primary effect, fluid volume transfer rate remained unaffected whereas Na transport rate changed abruptly in direct proportion to the Na concentration of the bathing media; (b) a secondary, delayed and partly reversible depression of fluid transfer rate following elevation of the NaCl concentration was observed only when the rate of transport was relatively high initially.
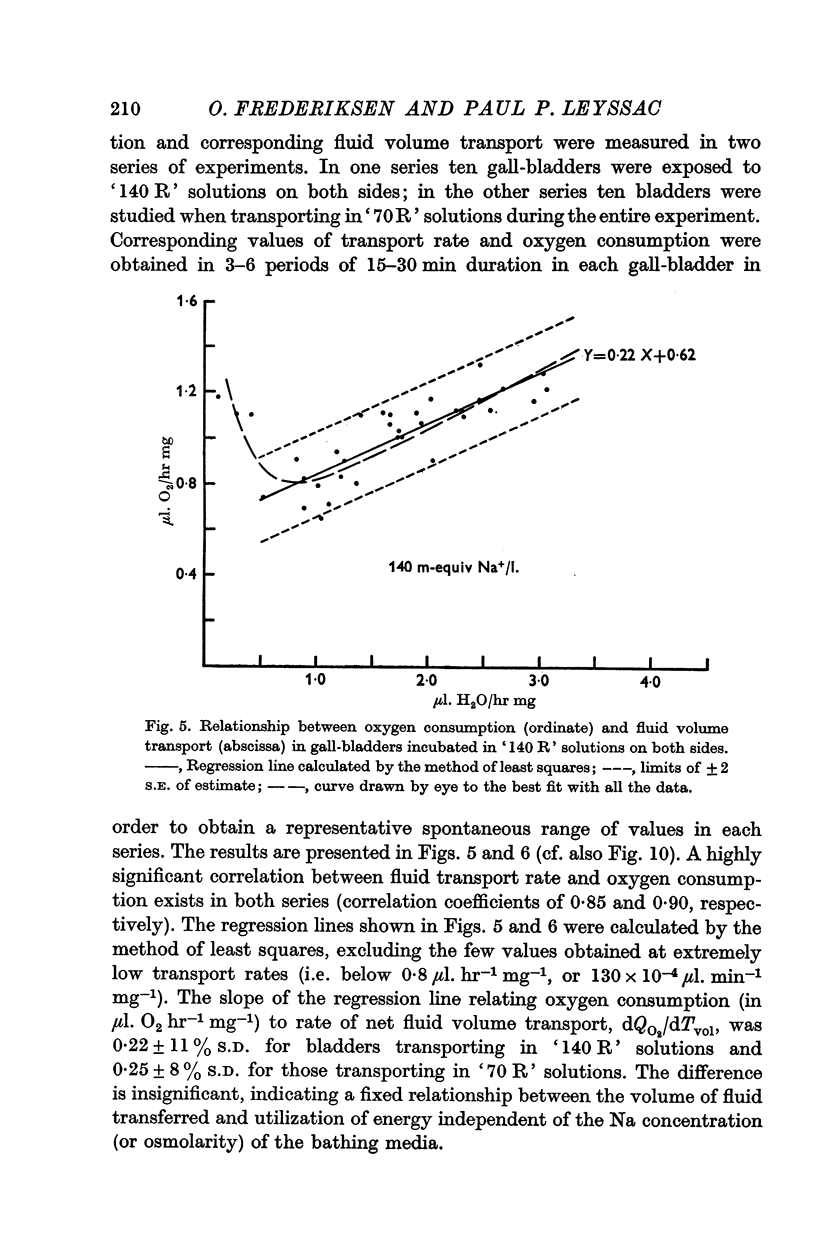
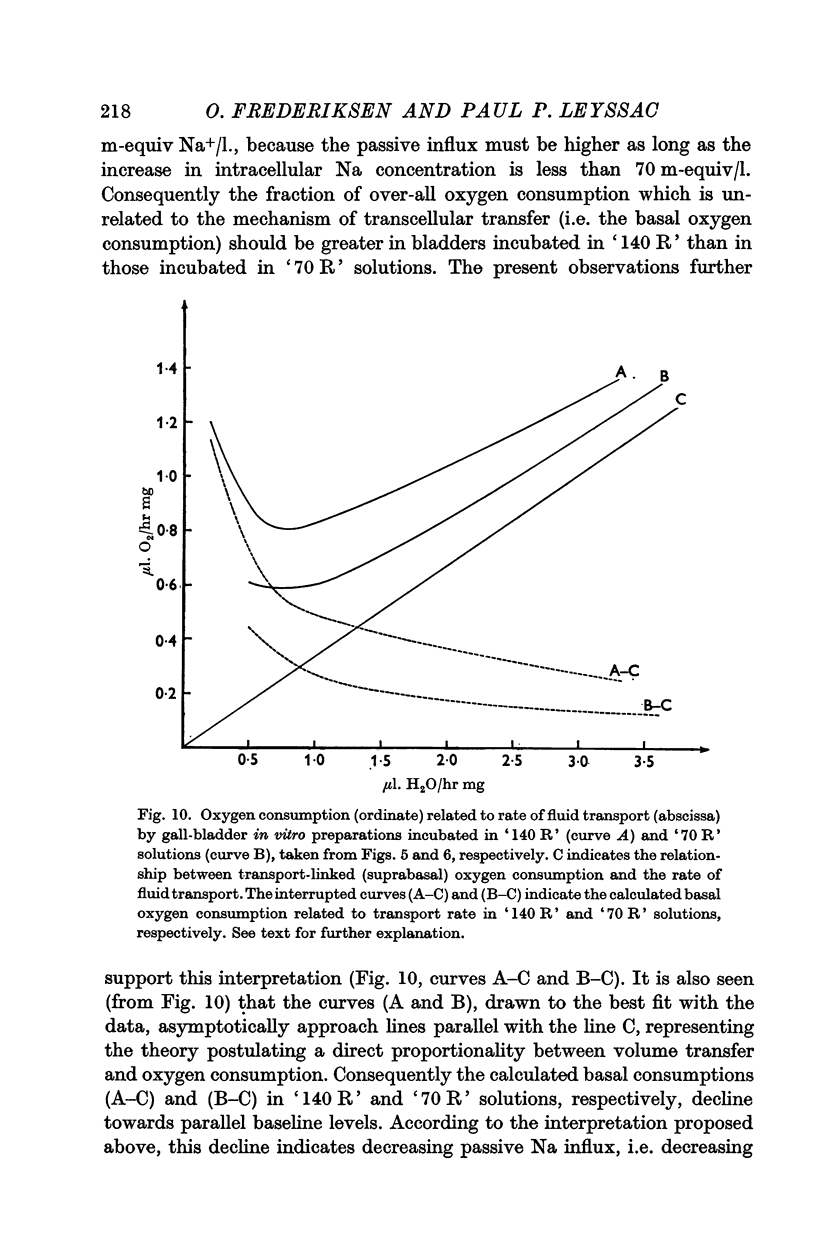
3. A fixed, and highly significant, linear relationship between changes in transport-linked oxygen consumption (ΔQO2) and measured net fluid volume transport (ΔTvol) was found independent of the NaCl concentration of the bathing media, dQO2/dTvol being 0·22 ± 11% and 0·25 ± 8% in bladders incubated in solutions containing 140 and 70 m-equiv Na+/l. respectively.
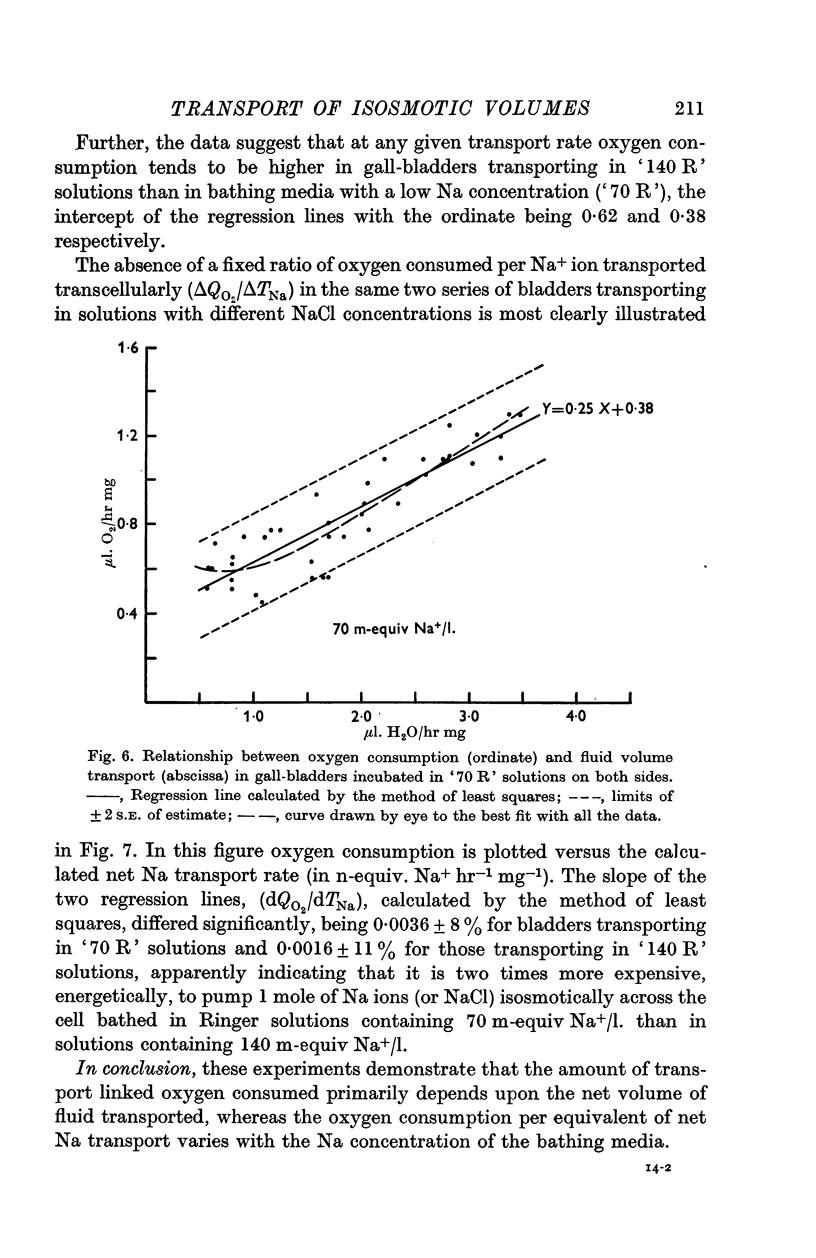
4. Oxygen consumption per equiv of Na+ (calculated) transported varied in inverse proportion to the Na concentration of the bathing media, dQO2/dTNa being 0·0016 ± 11% and 0·0036 ± 8% in `140 R' and `70 R' solutions, respectively.
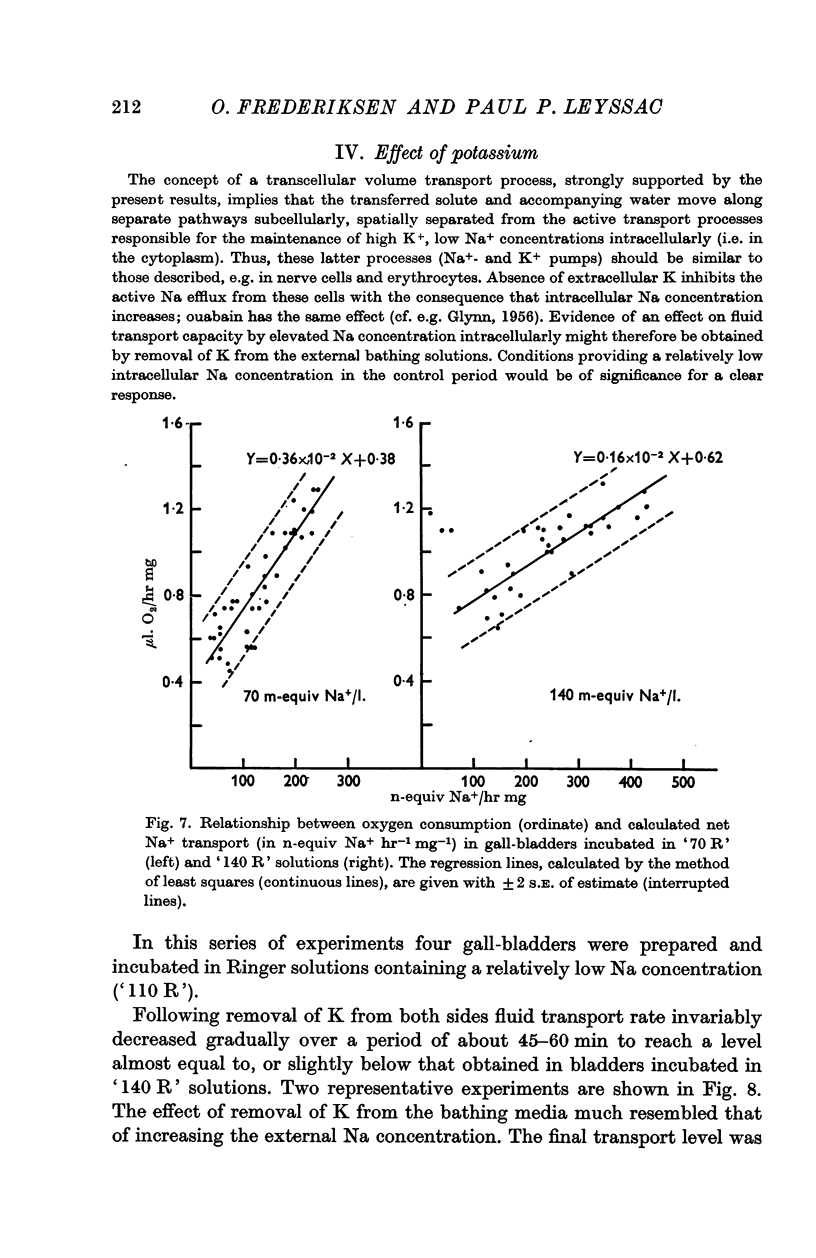
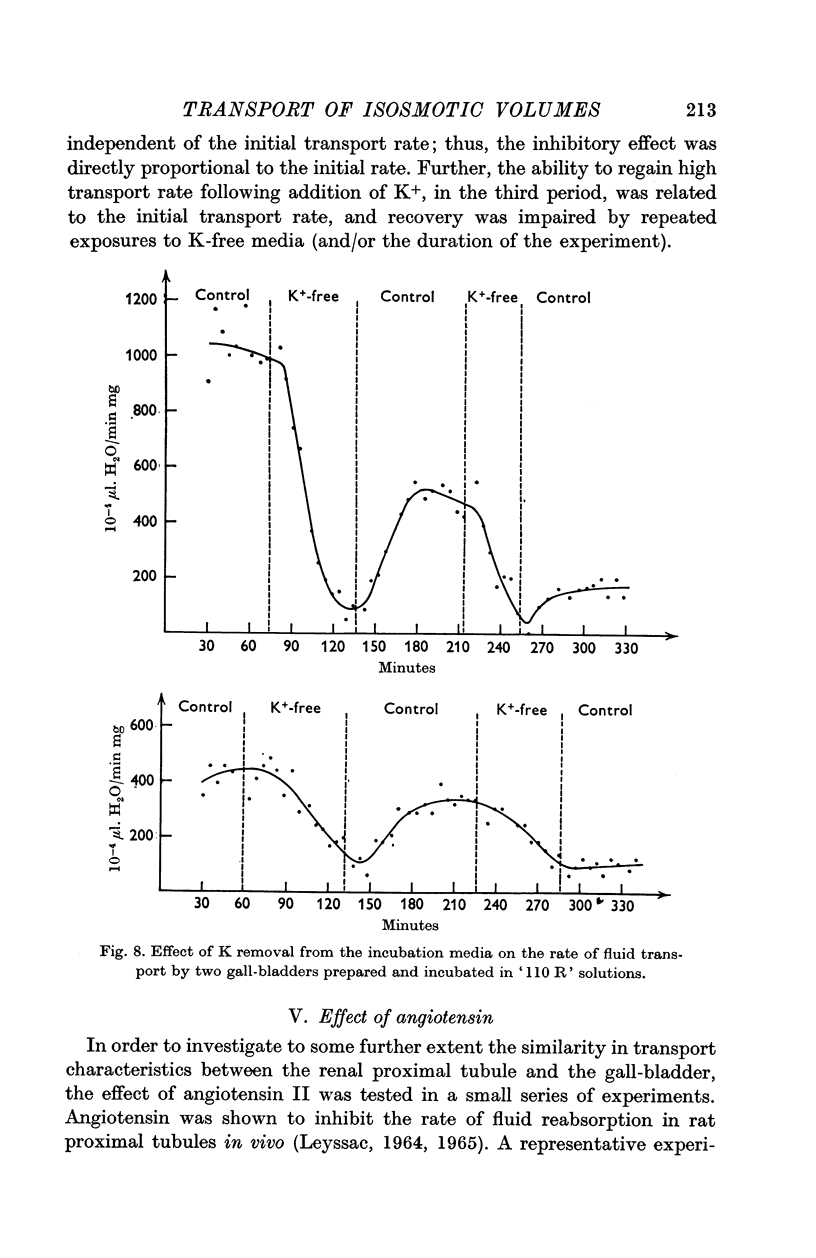
5. Removal of K from the bathing solutions was followed by a gradual and partly reversible depression of fluid transport rate to a minimum level (about 100 × 10-4 μl H2O. min-1.mg-1) independent of the initial transport rate.
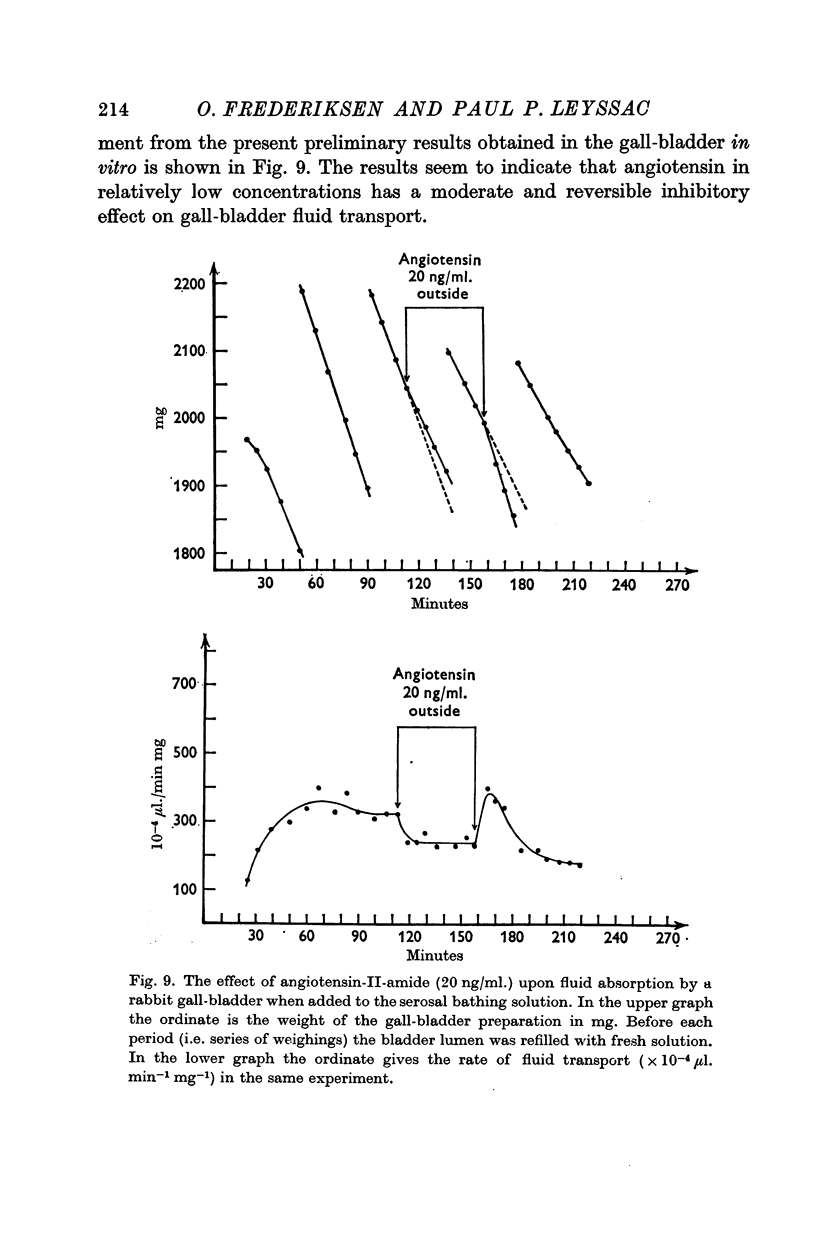
6. It is concluded that the range of absorption rates of isosmotic fluid from the gall-bladder lumen represents a range of energy requiring capacities for transfer of fluid volume units; the data suggest that the intracellular (cytoplasmic) ion composition, depending on the presence of external K, as well as hormonal action may influence the capacity of the transcellular fluid transport mechanism.
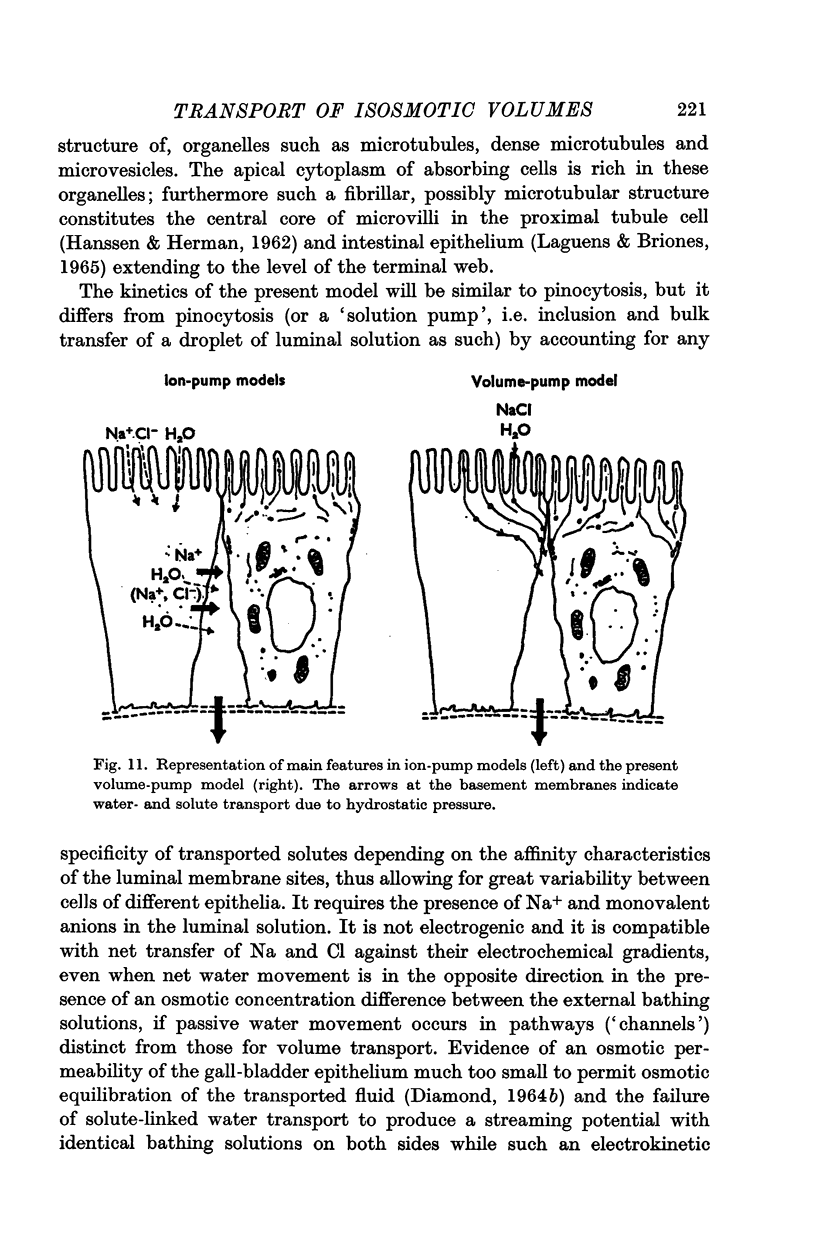
7. A model (a `mechanical volume pump') for transcellular transfer of fluid volume units, allowing for flexible specificity with regard to the actively transported solutes, and requiring the presence of Na+ and Cl-, is proposed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURRAN P. F. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol. 1960 Jul;43:1137–1148. doi: 10.1085/jgp.43.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T. W. The transport of salt and water across isolated rat ileum. Evidence for at least two distinct pathways. J Gen Physiol. 1967 Jan;50(3):695–727. doi: 10.1085/jgp.50.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of solute transport by the gall-bladder. J Physiol. 1962 May;161:474–502. doi: 10.1113/jphysiol.1962.sp006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of water transport by the gall-bladder. J Physiol. 1962 May;161:503–527. doi: 10.1113/jphysiol.1962.sp006900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The reabsorptive function of the gall-bladder. J Physiol. 1962 May;161:442–473. doi: 10.1113/jphysiol.1962.sp006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Tormey J. M. Role of long extracellular channels in fluid transport across epithelia. Nature. 1966 May 21;210(5038):817–820. doi: 10.1038/210817a0. [DOI] [PubMed] [Google Scholar]
- FISHER R. B. The absorption of water and of some small solute molecules from the isolated small intestine of the rat. J Physiol. 1955 Dec 29;130(3):655–664. doi: 10.1113/jphysiol.1955.sp005433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIM E. A MECHANISM FOR ABSORPTION OF SODIUM CHLORIDE SOLUTIONS FROM THE CANINE GALL BLADDER. Am J Physiol. 1963 Aug;205:247–254. doi: 10.1152/ajplegacy.1963.205.2.247. [DOI] [PubMed] [Google Scholar]
- GRIM E., SMITH G. A. Water flux rates across dog gallbladder wall. Am J Physiol. 1957 Dec;191(3):555–560. doi: 10.1152/ajplegacy.1957.191.3.555. [DOI] [PubMed] [Google Scholar]
- HANSSEN O. E., HERMAN L. The presence of an axial structure in the microvillus of the mouse convoluted proximal tubular cell. Lab Invest. 1962 Aug;11:610–616. [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol. 1961 Sep;45:143–179. doi: 10.1085/jgp.45.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- LEYSSAC P. P. THE IN VIVO EFFECT OF ANGIOTENSIN AND NORADRENALINE ON THE PROXIMAL TUBULAR REABSORPTION OF SALT IN MAMMALIAN KIDNEYS. Acta Physiol Scand. 1965 May-Jun;64:167–175. doi: 10.1111/j.1748-1716.1965.tb04165.x. [DOI] [PubMed] [Google Scholar]
- LEYSSAC P. P. THE IN VIVO EFFECT OF ANGIOTENSIN ON THE PROXIMAL TUBULAR REABSORPTION OF SALT IN RAT KIDNEYS. Acta Physiol Scand. 1964 Dec;62:436–448. doi: 10.1111/j.1748-1716.1964.tb10441.x. [DOI] [PubMed] [Google Scholar]
- Laguens R., Briones M. Fine structure of the microvillus of columnar epithelium cells of human intestine. Lab Invest. 1965 Sep;14(9):1616–1623. [PubMed] [Google Scholar]
- Martin D. W., Diamond J. M. Energetics of coupled active transport of sodium and chloride. J Gen Physiol. 1966 Nov;50(2):295–315. doi: 10.1085/jgp.50.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIDOT A. L., DIAMOND J. M. STREAMING POTENTIALS IN A BIOLOGICAL MEMBRANE. Nature. 1964 Feb 15;201:701–702. doi: 10.1038/201701a0. [DOI] [PubMed] [Google Scholar]
- Sandborn E., Szeberenyi A., Messier P. E., Bois P. A new membrane model derived from a study of filaments, microtubules and membranes. Rev Can Biol. 1965 Dec;24(4):243–276. [PubMed] [Google Scholar]
- Tormey J. M., Diamond J. M. The ultrastructural route of fluid transport in rabbit gall bladder. J Gen Physiol. 1967 Sep;50(8):2031–2060. doi: 10.1085/jgp.50.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER H. O. TRANSPORT OF ELECTROLYTES AND WATER ACROSS WALL OF RABBIT GALL BLADDER. Am J Physiol. 1963 Sep;205:427–438. doi: 10.1152/ajplegacy.1963.205.3.427. [DOI] [PubMed] [Google Scholar]
- WHITLOCK R. T., WHEELER H. O. COUPLED TRANSPORT OF SOLUTE AND WATER ACROSS RABBIT GALLBLADDER EPITHELIUM. J Clin Invest. 1964 Dec;43:2249–2265. doi: 10.1172/JCI105099. [DOI] [PMC free article] [PubMed] [Google Scholar]


