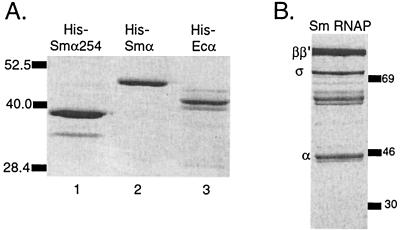
FIG. 3.
Analysis of six-His-tagged purified α-subunits resolved by SDS-PAGE and stained with Coomassie blue R250. (A) S. meliloti (Sm) αΔ254 purified in batch on Ni+2 resin under native conditions from cells containing pMP21 (lane 1), six-His-tagged S. meliloti α purified in batch on Ni+2 resin under denaturing conditions from cells containing pMP19a (lane 2), and six-His-tagged E. coli α purified under denaturing conditions on a Ni+2 spin column from cells containing pHTT7α (lane 3). Six-His-tagged S. meliloti α has a larger molecular mass than untagged α due to the addition of 62 amino acids at the N terminus of the six-His-tagged protein (see Materials and Methods). (B) RNAP isolated from S. meliloti. Size markers are in kilodaltons.