Abstract
1. The patterns of medial gastrocnemius (MG) motor unit firing in response to MG muscle stretch have been studied in decerebrate cats, using intracellular recording techniques. In most of the units, the motoneurone axonal conduction velocity and cell input resistance were measured, and the maximum amplitude and wave form of the group Ia composite EPSP evoked by MG nerve stimulation were determined. The mechanical properties of the muscle unit portion of each motor unit were also studied.
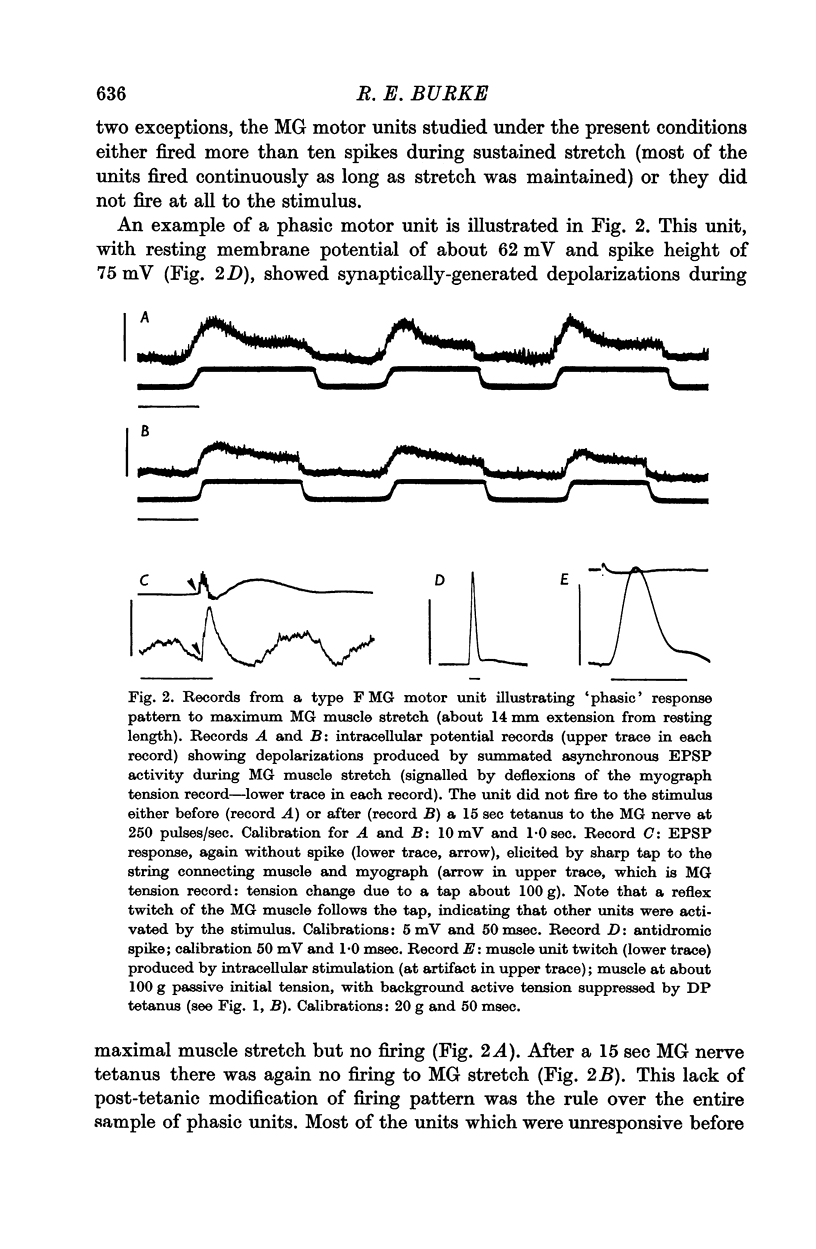
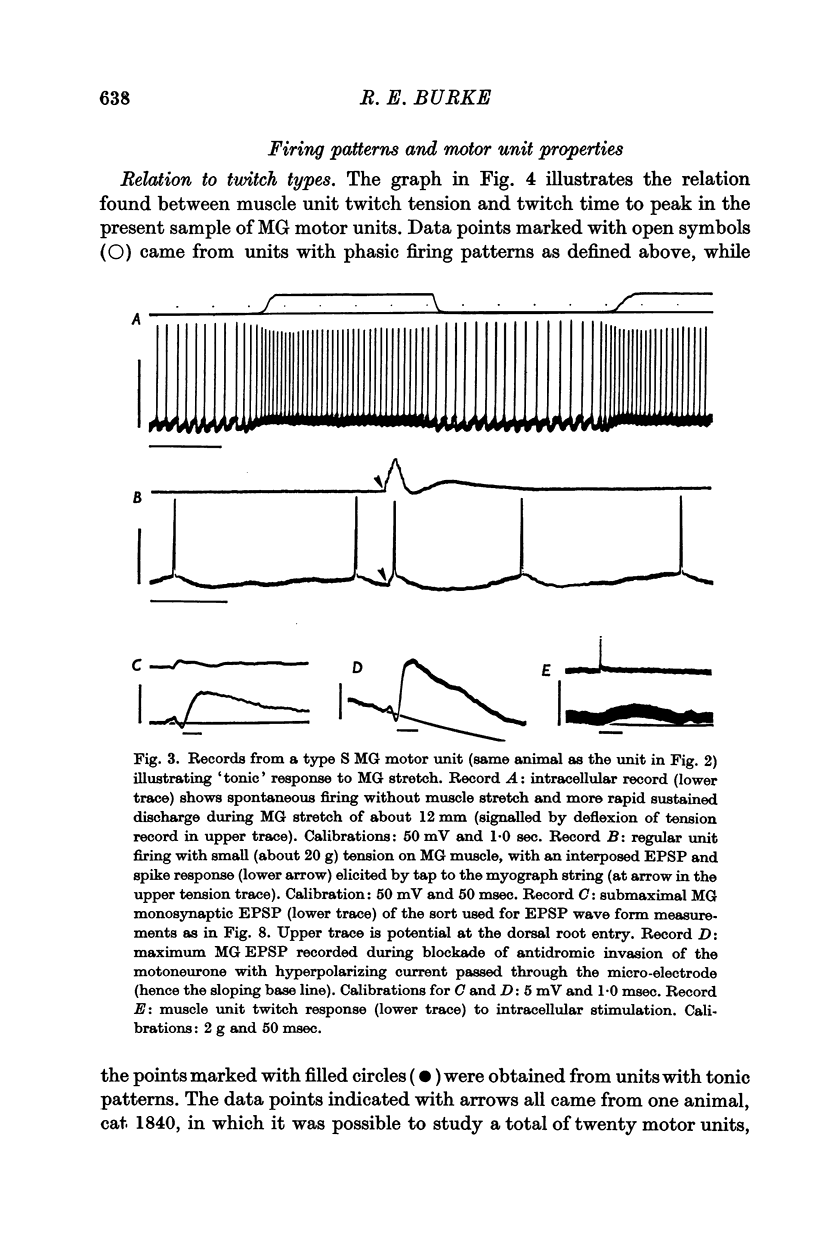
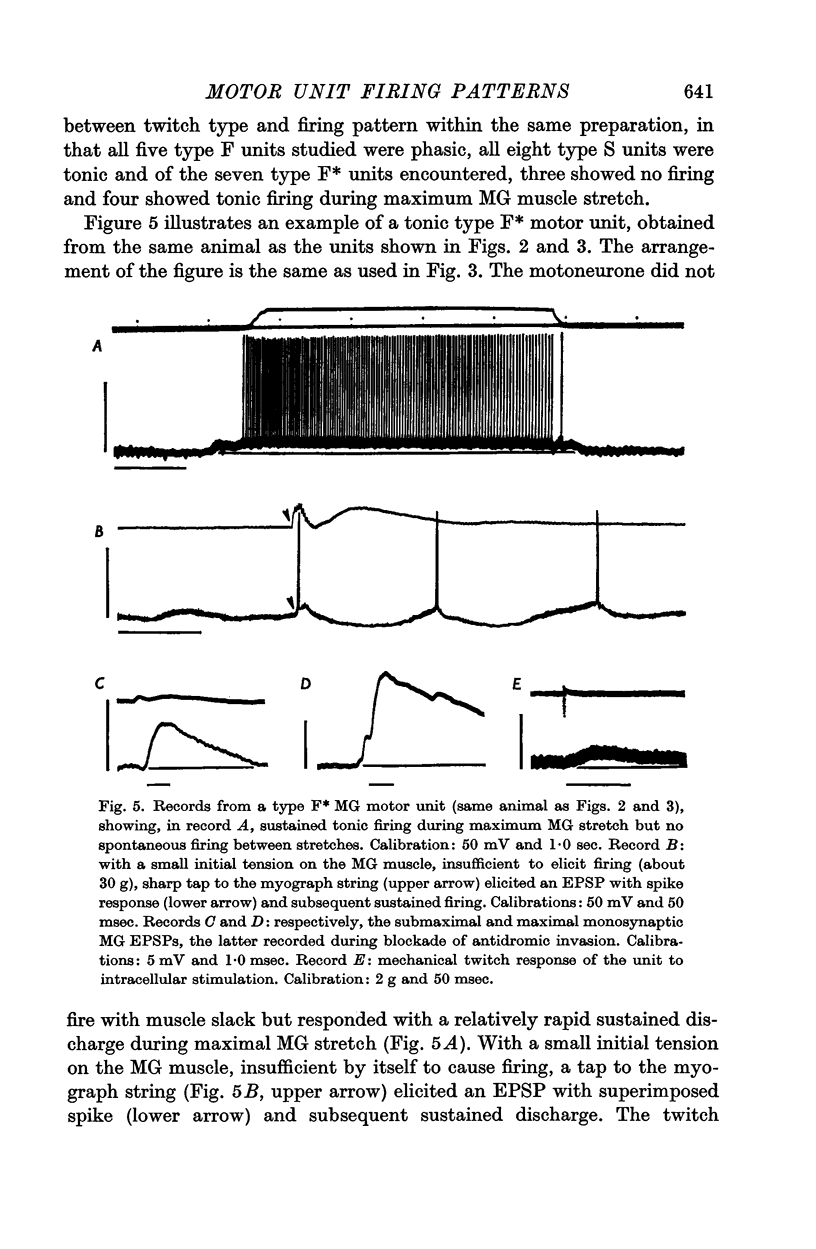
2. Motor unit firing patterns were classified into two groups, `tonic' and `phasic'. With few exceptions, tonic motor units showed sustained firing throughout MG stretches of varying duration, while most phasic units did not fire at all to the same stimulus. Tetanization of the afferents did not convert any of the previously phasic units to tonic firing.
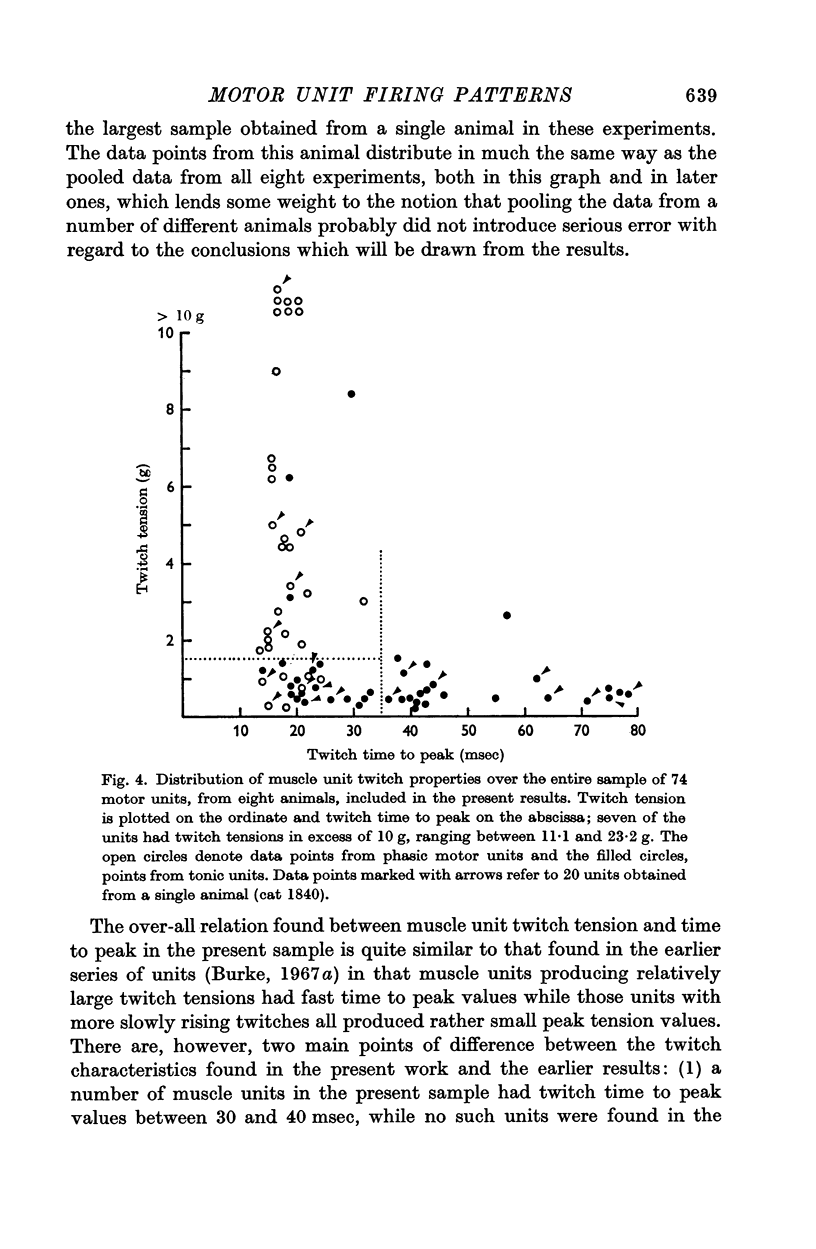
3. MG motor units in this study were divided into three groups on the basis of the muscle unit twitch properties. Units with short twitch time to peak values (< 35 msec) were subdivided into two groups according to twitch tension output: (a) type F, with twitch tension > 1·5 g, and (b) type F*, with twitch tension < 1·5 g. Units with slow twitch time to peak (> 35 msec) were classified as type S.
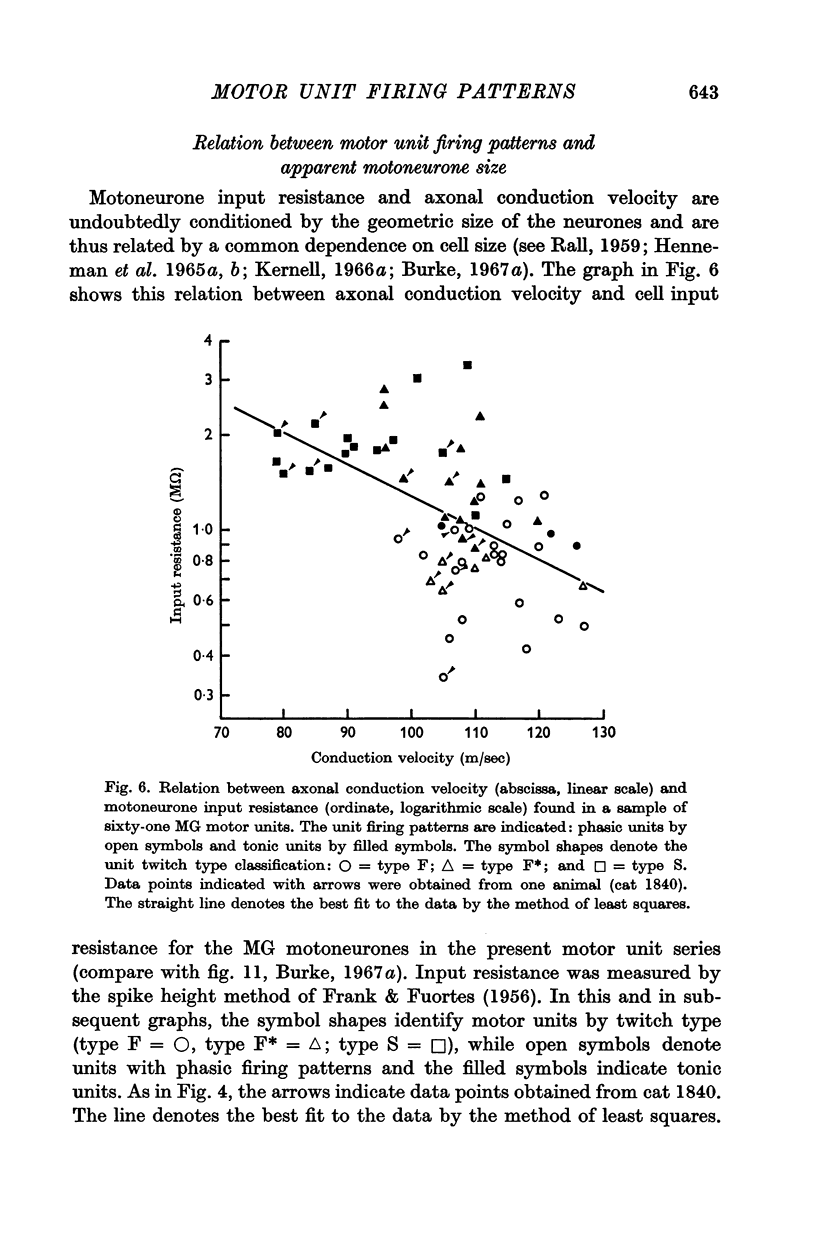
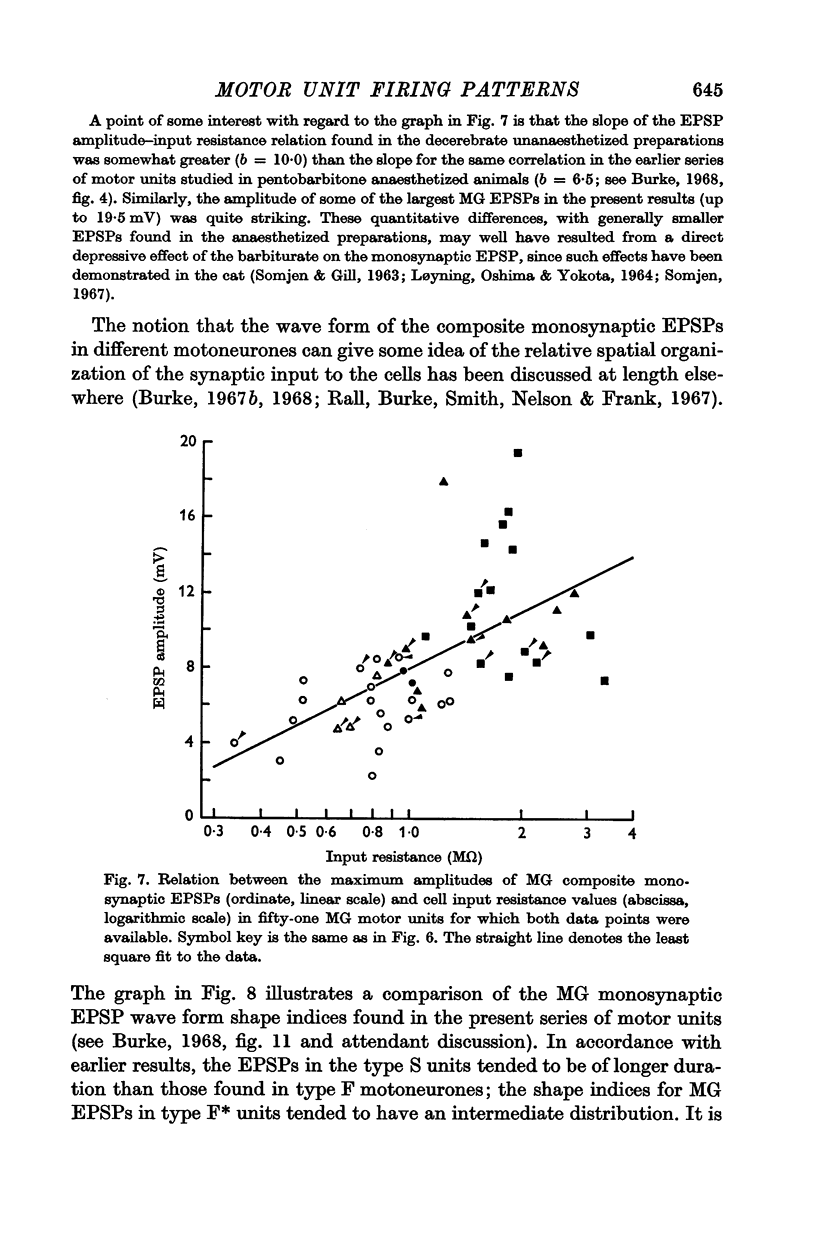
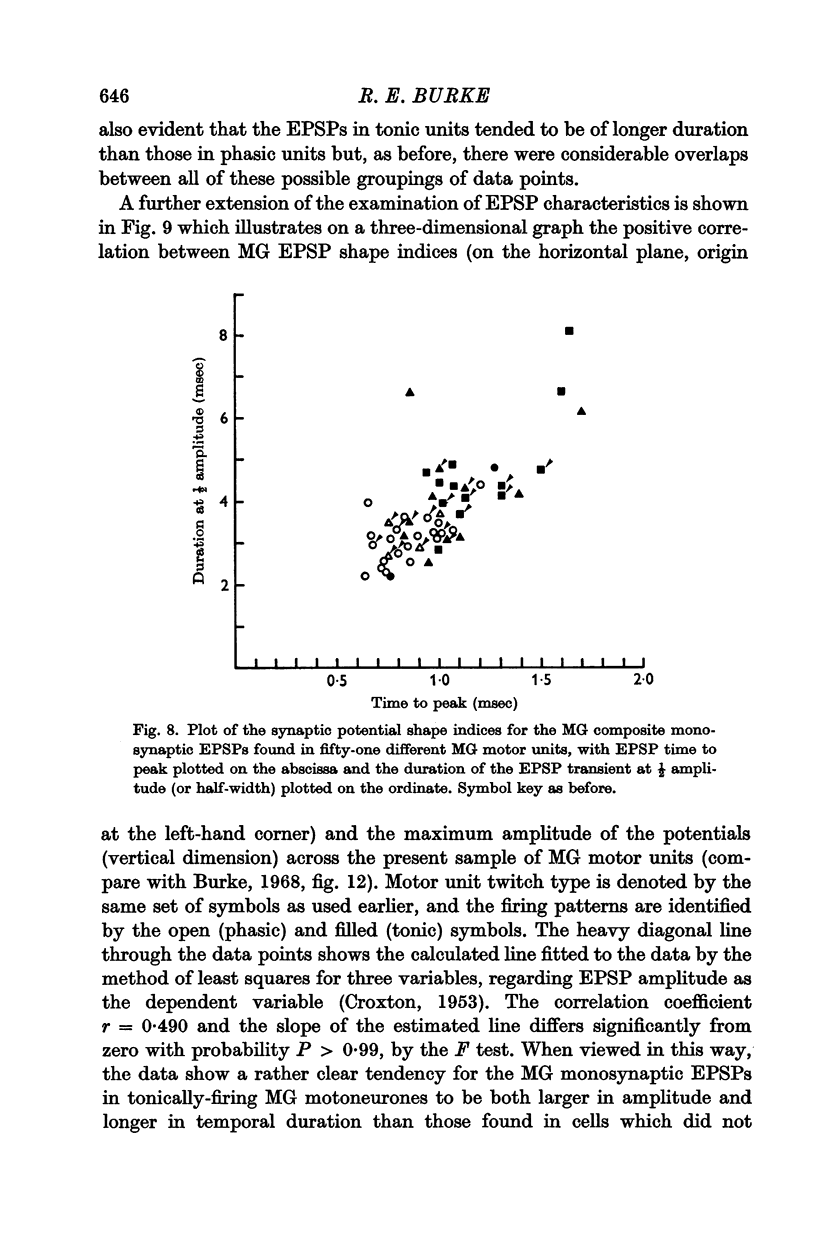
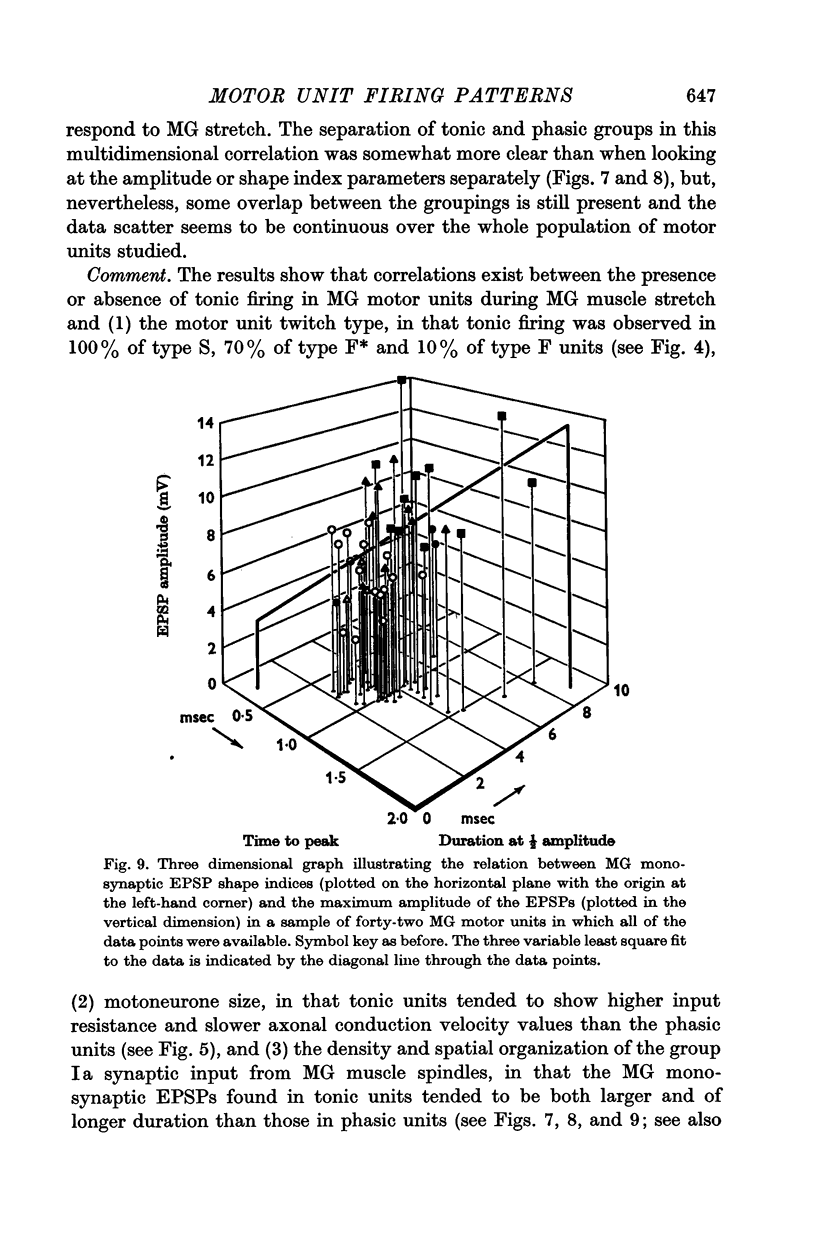
4. The presence or absence of tonic firing during sustained MG stretch was found to be significantly related to the following factors: (a) the motor unit twitch type, in that tonic firing was observed in 100% of type S, 70% of type F*, and only 10% of type F units; (b) the apparent motoneurone size, in that tonic units tended to have higher cell input resistance values and slower axonal conduction velocities than phasic units; and (c) the density and spatial organization of the group Ia synaptic input, in that the MG monosynaptic EPSPs found in tonic motor units tended to be both larger in amplitude and longer in duration than those found in phasic units.
5. The intrinsic properties of MG motor units, the characteristics of the group Ia synaptic input to the motoneurones and the unit firing patterns elicited by MG muscle stretch appear to be mutually interrelated, forming a pattern of motor units within the MG pool which is analogous to the pattern thought to characterize the motor units belonging to `slow' and `fast' muscles. In most respects this pattern of MG motor units appears to be a continuum without clearly separable subgroups.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The generation of impulses in motoneurones. J Physiol. 1957 Dec 3;139(2):232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANANDAN M. S., ECCLES R. M., WESTERMAN R. A. SINGLE MOTOR UNITS OF MAMMALIAN MUSCLE. J Physiol. 1965 May;178:359–367. doi: 10.1113/jphysiol.1965.sp007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., FRANK K., BECKER M. C. Steps in the production of motoneuron spikes. J Gen Physiol. 1957 May 20;40(5):735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., KELLERTH J. O., WILLIAMS T. D. INTRACELLULAR ASPECTS OF STIMULATING MOTONEURONES BY MUSCLE STRETCH. J Physiol. 1964 Nov;174:435–452. doi: 10.1113/jphysiol.1964.sp007496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- Granit R., Kernell D., Lamarre Y. Algebraical summation in synaptic activation of motoneurones firing within the 'primary range' to injected currents. J Physiol. 1966 Nov;187(2):379–399. doi: 10.1113/jphysiol.1966.sp008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENATSCH H. D., SCHULTE F. J., BUSCH G. [Modification of the tonic-phasic reaction type of various extensor motor neurons by variation of their inflow]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1959;270:161–173. [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- KOLMODIN G. M., SKOGLUND C. R. Slow membrane potential changes accompanying excitation and inhibition in spinal moto- and interneurons in the cat during natural activation. Acta Physiol Scand. 1958 Oct 28;44(1):11–54. doi: 10.1111/j.1748-1716.1958.tb01607.x. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- SANDOW A. POTENTIATION OF MUSCULAR CONTRACTION. Arch Phys Med Rehabil. 1964 Feb;45:62–81. [PubMed] [Google Scholar]
- SASAKI K., OTANI T. Accommodation in spinal motoneurons of the cat. Jpn J Physiol. 1961 Aug 15;11:443–456. doi: 10.2170/jjphysiol.11.443. [DOI] [PubMed] [Google Scholar]
- SASAKI K., TANAKA T. PHASIC AND TONIC INNERVATION OF SPINAL ALPHA MOTONEURONS FROM UPPER BRAIN CENTERS. Jpn J Physiol. 1964 Feb 15;14:56–66. doi: 10.2170/jjphysiol.14.56. [DOI] [PubMed] [Google Scholar]
- SOMJEN G. G., GILL M. The mechanism of the blockade of synaptic transmission in the mammalian spinal cord by diethyl ether and by thiopental. J Pharmacol Exp Ther. 1963 Apr;140:19–30. [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- Ushiyama J., Koizumi K., Brooks C. M. Accommodative reactions of neuronal elements in the spinal cord. J Neurophysiol. 1966 Nov;29(6):1028–1045. doi: 10.1152/jn.1966.29.6.1028. [DOI] [PubMed] [Google Scholar]
- Yamashita H. Factors which decide the excitability of a spinal motoneuron in the cat. Jpn J Physiol. 1966 Dec 15;16(6):684–701. doi: 10.2170/jjphysiol.16.684. [DOI] [PubMed] [Google Scholar]


