Abstract
1. Measurements were made of milk yield, mammary blood flow and mammary arteriovenous differences during the measurement of substrate entry rate by the isotope dilution method using [U-14C]glucose, acetate, palmitate, stearate or oleate in conscious lactating goats after 24 hr starvation.
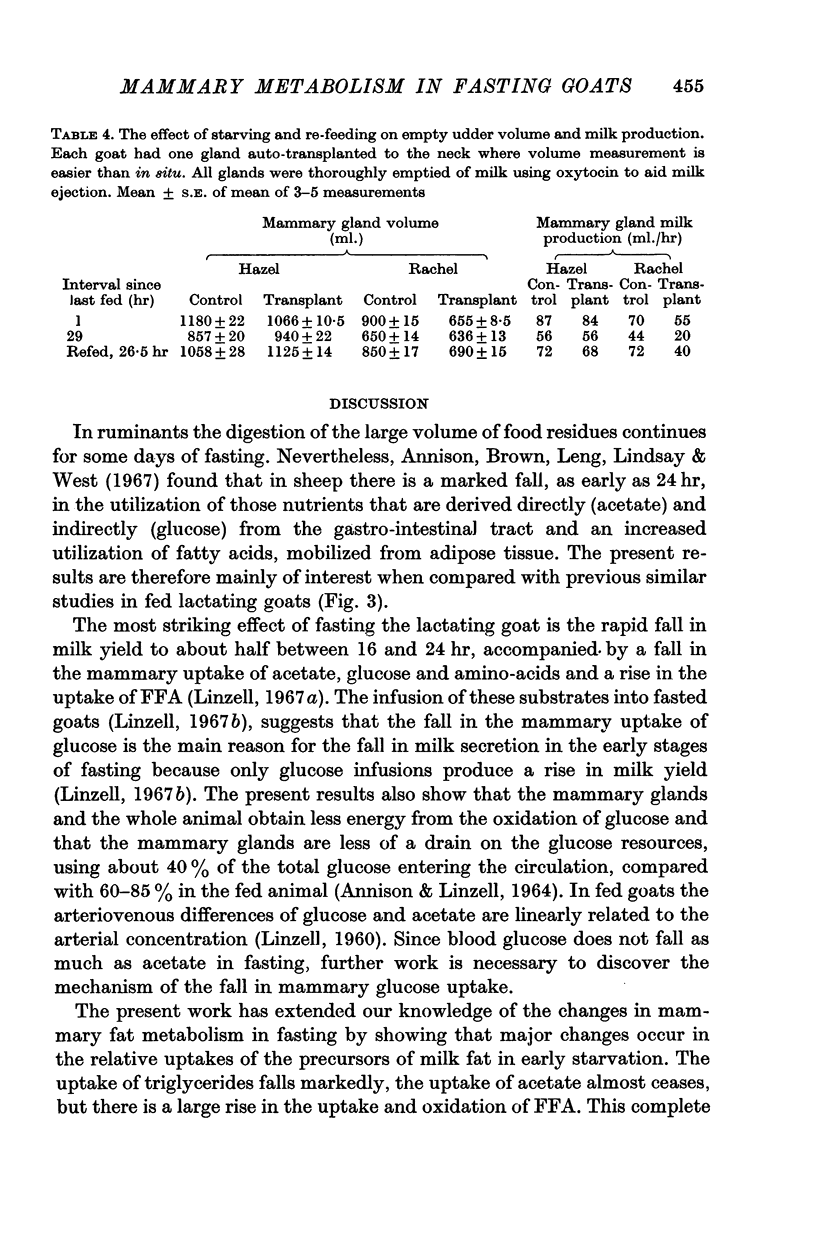
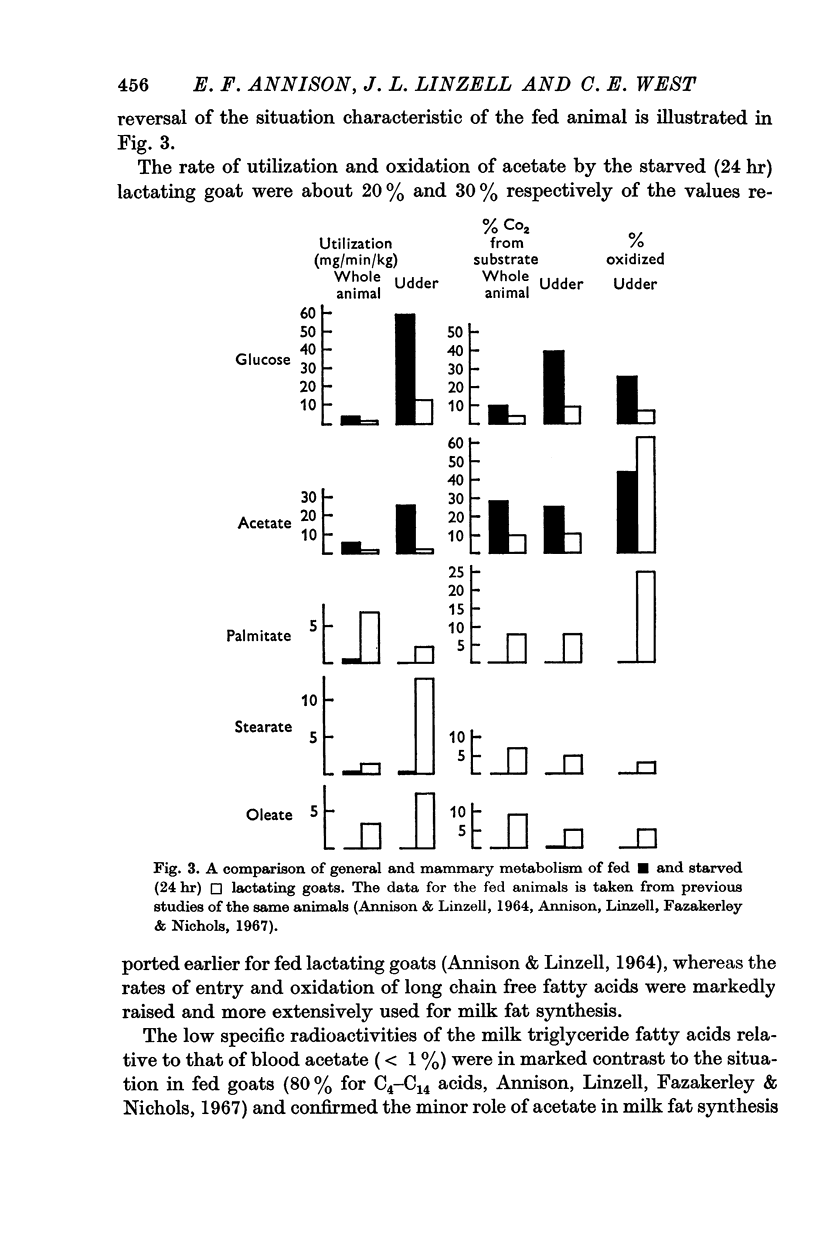
2. As previously reported, in fasting, milk yield fell to 40 ± 3·4 (S.E.)%, lactose secretion to 31 ± 3·4%, milk fat secretion to 81 ± 6·7% and mammary blood flow fell to 53 ± 7·5% of the values before fasting. Mammary O2 uptake was only 45 ± 5% of the mean value in fed animals and there were marked falls in the uptakes of glucose, acetate and triglycerides, a smaller fall in β-hydroxybutyrate uptake, and a large increase in free fatty acid uptake.
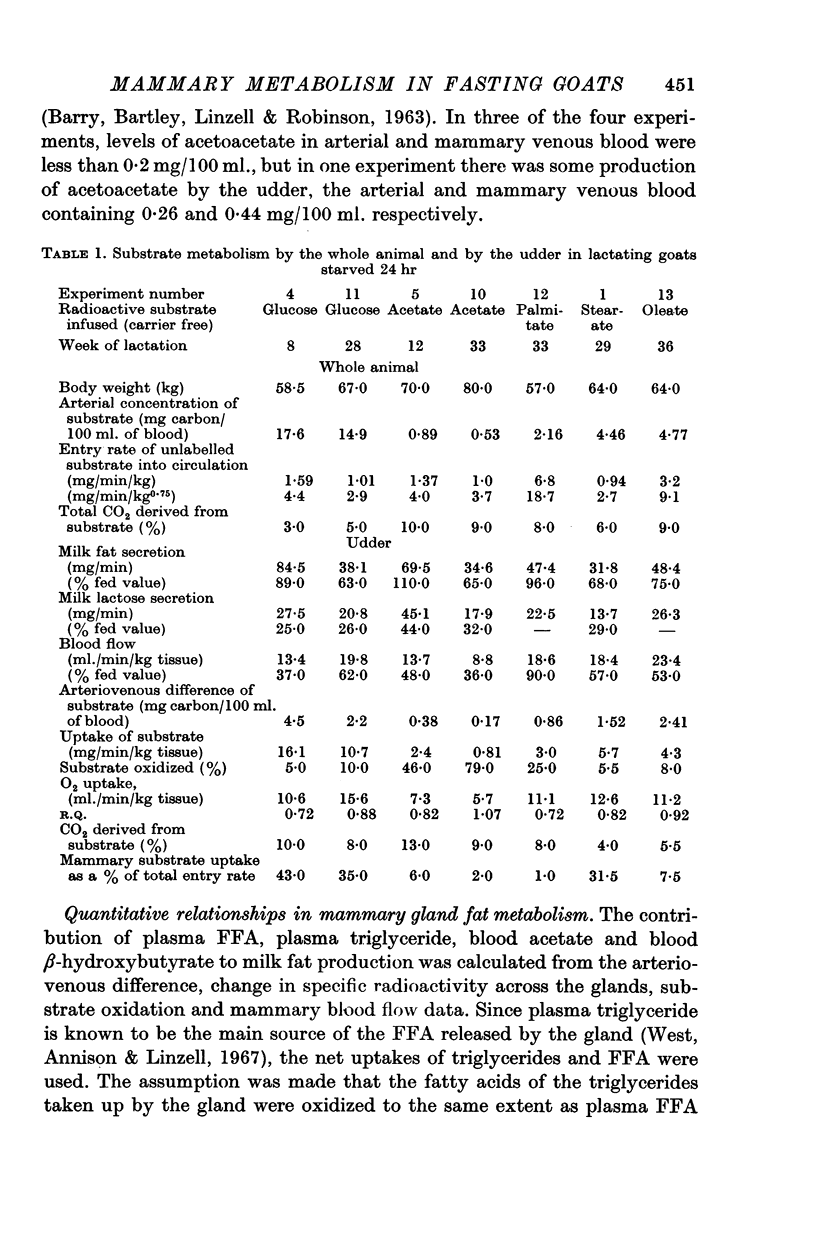
3. Glucose was found to enter the circulation of the fasting animal at 1-1·6 mg/min/kg body wt. (entry rate) and it gave rise to 3-5% of the total CO2. The udder took up 10·7-16·1 mg/min/kg of tissue and 8-10% of mammary CO2 was derived from glucose, although only 5-10% was oxidized. Mammary uptake accounted for 35-43% of the total glucose entering the circulation.
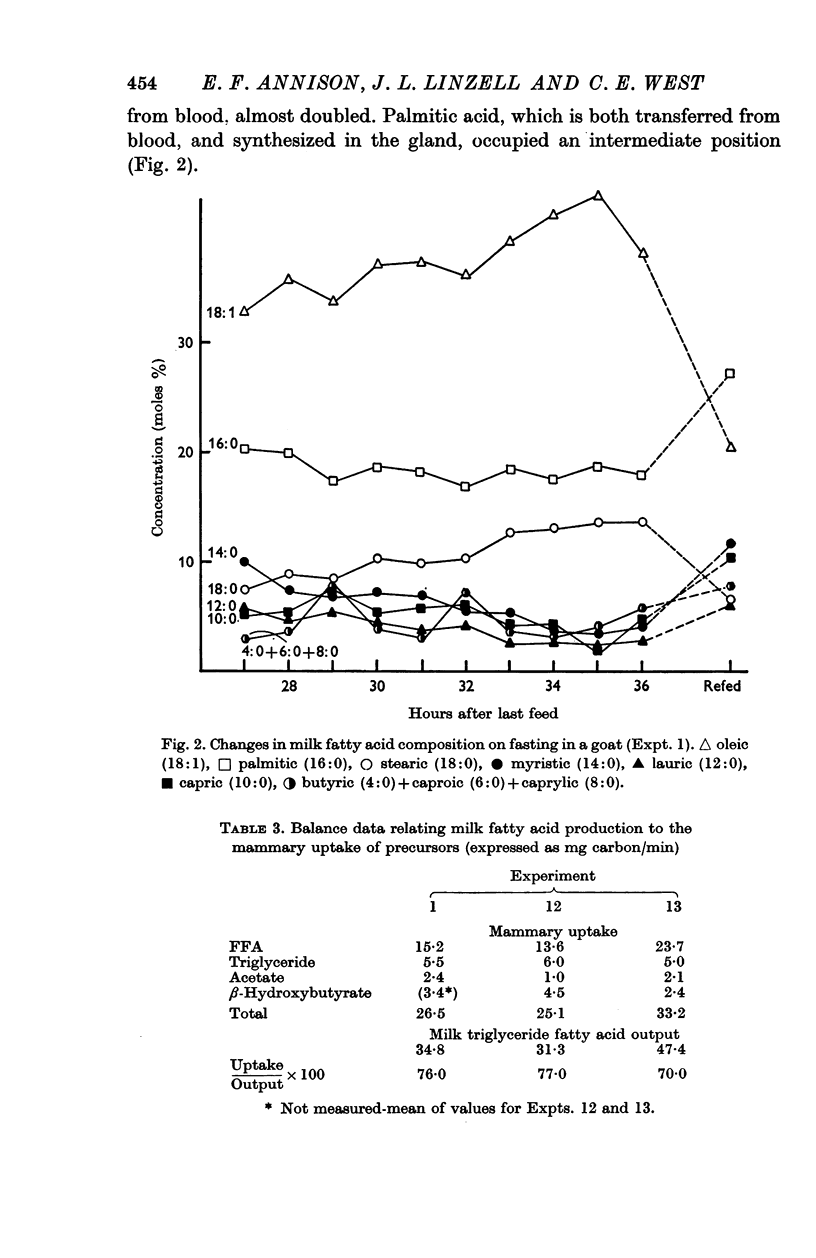
4. In the whole animal acetate entry rate was 1-1·4 mg/min/kg and 9-10% of total CO2 was derived from it. The udder used 0·8-2·4 mg/min/kg of tissue and 9-13% of mammary CO2 was derived from acetate, 46-79% of that taken up being oxidized. Mammary uptake accounted for only 2-6% of the total acetate entry rate. Negligible quantities of isotope were found in milk fatty acids and there was a fall in the proportion of milk fatty acids of chain length up to C14 which in fed animals are synthesized from acetate and β-hydroxybutyrate.
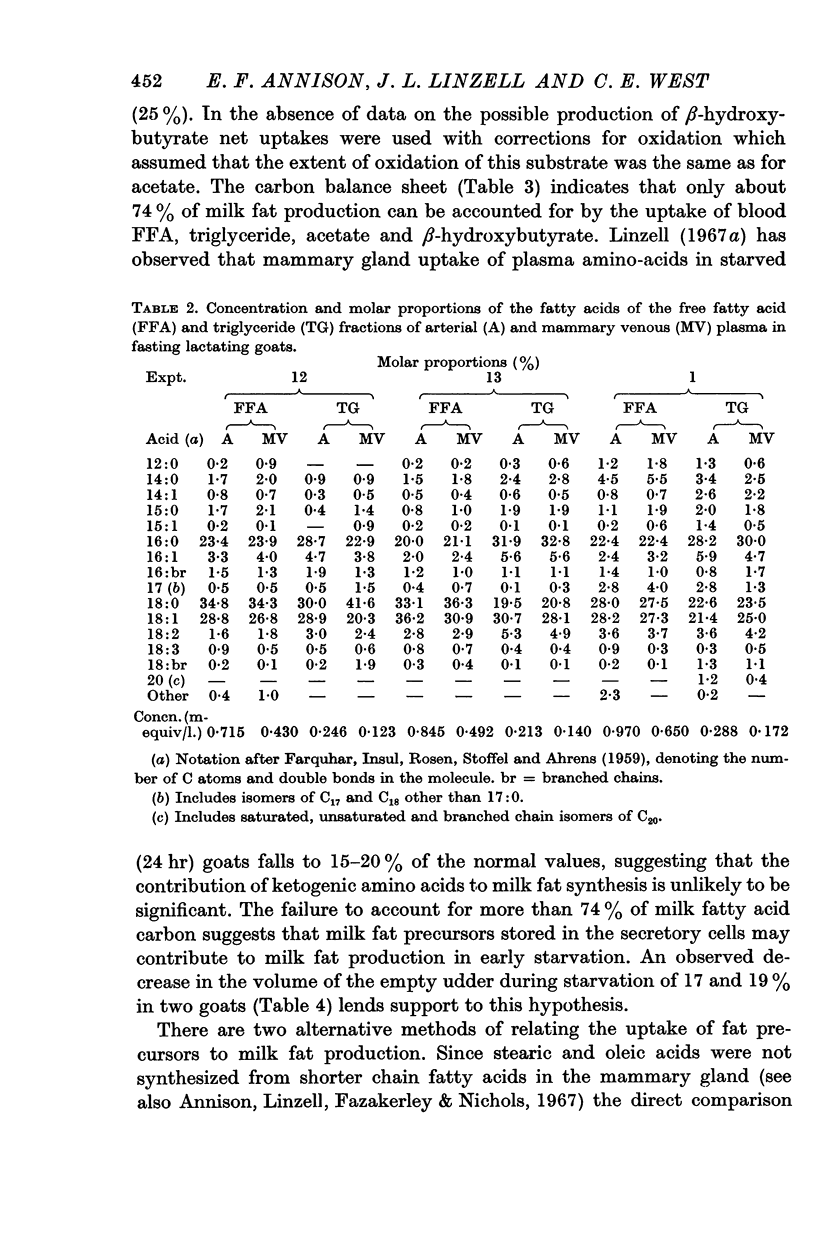
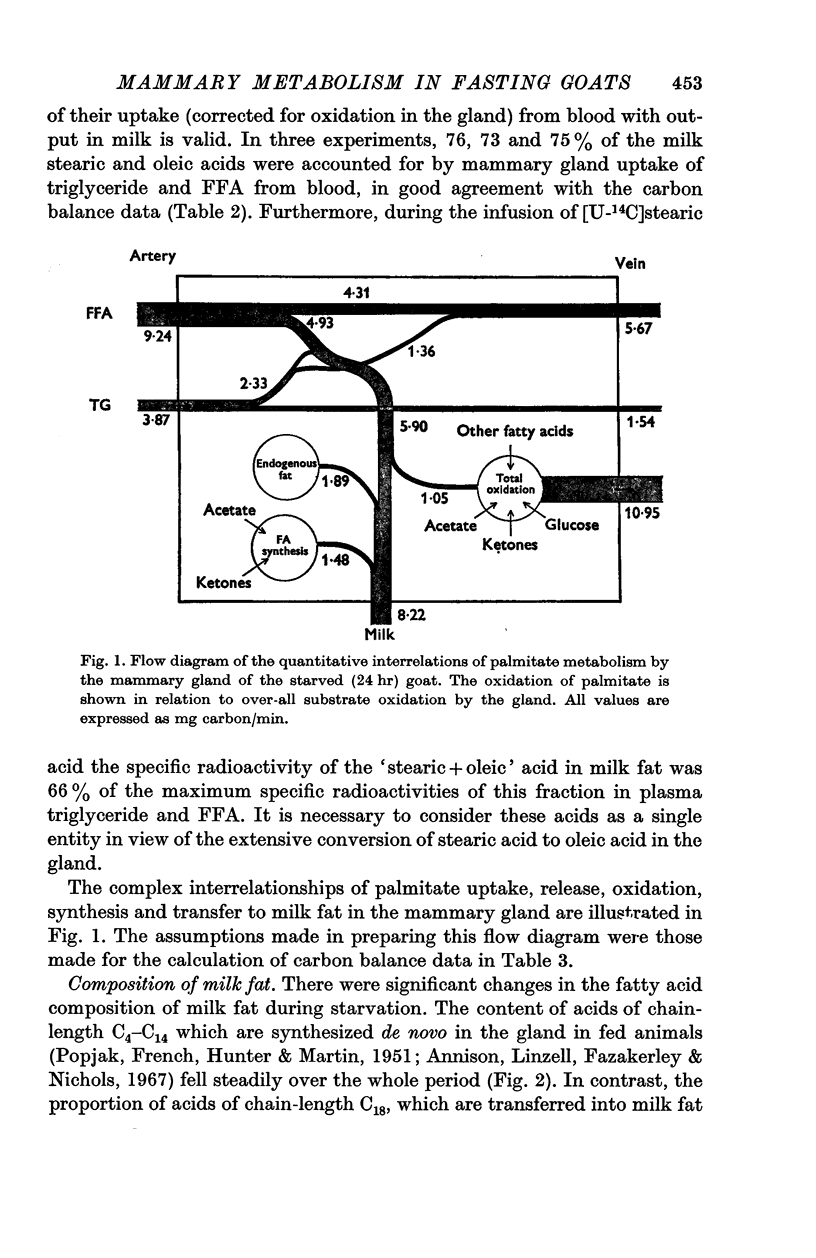
5. Palmitate, stearate and oleate entered the circulation as free fatty acids at 0·94-6·8 mg/min/kg and 6-9% of total CO2 was derived from each. The udder took up 3·0-5·7 mg/min/kg of tissue and 4-8% of mammary CO2 was derived from each acid. In the udder 8 and 5·5% of stearate and oleate were oxidized and 25% of palmitate. Mammary uptake of stearate was 31·5% of the total entry rate, palmitate 1%, and oleate 7·5%. Only long chain milk fatty acids were labelled.
6. During fasting the mammary R.Q. was 0·85 ± 0·045 compared with a value in fed animals of 1·24 ± 0·02, when the udder is synthesizing fatty acids from acetate. The total mammary uptake of lipid precursors was only 74% of the rate of milk fat secretion and there was an 18% shrinkage in empty udder volume, suggesting the use of endogenous mammary tissue substrates.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AL-KASSAB S., BOYLAND E., WILLIAMS K. An enzyme from rat liver catalysing conjugations with glutathione. 2. Replacement of nitro groups. Biochem J. 1963 Apr;87:4–9. doi: 10.1042/bj0870004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., LINZELL J. L. THE OXIDATION AND UTILIZATION OF GLUCOSE AND ACETATE BY THE MAMMARY GLAND OF THE GOAT IN RELATION TO THEIR OVER-ALL METABOLISM AND MILK FORMATION. J Physiol. 1964 Dec;175:372–385. doi: 10.1113/jphysiol.1964.sp007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., WHITE R. R. Glucose utilization in sheep. Biochem J. 1961 Jul;80:162–169. doi: 10.1042/bj0800162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annison E. F., Brown R. E., Leng R. A., Lindsay D. B., West C. E. Rates of entry and oxidation of acetate, glucose, D(-)-beta-hydroxybutyrate, palmitate, oleate and stearate, and rates of production and oxidation of propionate and butyrate in fed and starved sheep. Biochem J. 1967 Jul;104(1):135–147. doi: 10.1042/bj1040135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annison E. F., Linzell J. L., Fazakerley S., Nichols B. W. The oxidation and utilization of palmitate, stearate, oleate and acetate by the mammary gland of the fed goat in relation to their overall metabolism, and the role of plasma phospholipids and neutral lipids in milk-fat synthesis. Biochem J. 1967 Mar;102(3):637–647. doi: 10.1042/bj1020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY J. M., BARTLEY W., LINZELL J. L., ROBINSON D. S. THE UPTAKE FROM THE BLOOD OF TRIGLYCERIDE FATTY ACIDS OF CHYLOMICRA AND LOW-DENSITY LIPOPROTEINS BY THE MAMMARY GLAND OF THE GOAT. Biochem J. 1963 Oct;89:6–11. doi: 10.1042/bj0890006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR J. W., INSULL W., Jr, ROSEN P., STOFFEL W., AHRENS E. H., Jr The analysis of fatty acid mixtures by gas-liquid chromatography; construction and operation of an ionization chamber instrument. Nutr Rev. 1959 Aug;17(8 Suppl):1–30. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HITCHCOCK C., JAMES A. T. OXIDATION OF UNSATURATED FATTY ACIDS BY LEAF TISSUE. J Lipid Res. 1964 Oct;5:593–599. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- JONES G. B. DETERMINATION OF THE SPECIFIC ACTIVITY OF LABELED BLOOD GLUCOSE BY LIQUID SCINTILLATION USING GLUCOSE PENTAACETATE. Anal Biochem. 1965 Aug;12:249–258. doi: 10.1016/0003-2697(65)90088-6. [DOI] [PubMed] [Google Scholar]
- LINZELL J. L. Mammary-gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. J Physiol. 1960 Oct;153:492–509. doi: 10.1113/jphysiol.1960.sp006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Annison E. F., Fazakerley S., Leng R. A. The incorporation of acetate, stearate and D(-)-beta-hydroxybutyrate into milk fat by the isolated perfused mammary gland of the goat. Biochem J. 1967 Jul;104(1):34–42. doi: 10.1042/bj1040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. Infusion and blood sampling techniques for use in minimally restrained goats. J Physiol. 1966 Oct;186(2):79P–81P. [PubMed] [Google Scholar]
- Linzell J. L. Measurement of udder volume in live goats as an index of mammary growth and function. J Dairy Sci. 1966 Mar;49(3):307–311. doi: 10.3168/jds.S0022-0302(66)87853-0. [DOI] [PubMed] [Google Scholar]
- Linzell J. L. The effect of infusions of glucose, acetate and amino acids on hourly milk yield in fed, fasted and insulin-treated goats. J Physiol. 1967 May;190(2):347–357. doi: 10.1113/jphysiol.1967.sp008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. The effect of very frequent milking and of oxytocin on the yield and composition of milk in fed and fasted goats. J Physiol. 1967 May;190(2):333–346. doi: 10.1113/jphysiol.1967.sp008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPJAK G., FRENCH T. H., HUNTER G. D., MARTIN A. J. P. Mode of formation of milk fatty acids from acetate in the goat. Biochem J. 1951 May;48(5):612–618. doi: 10.1042/bj0480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. E., Rowbotham T. R. The use of a computer in the determination by gas-liquid chromatography of the concentration and identification of individual fatty acids present as free fatty acids, triglycerides and cholesteryl esters. J Chromatogr. 1967 Sep;30(1):62–76. doi: 10.1016/s0021-9673(00)84113-8. [DOI] [PubMed] [Google Scholar]


