Fig. 3. Intact GTP-loading of Rac and coupling to ELMO1 and CrkII by the DHR-1 mutant of DOCK180.
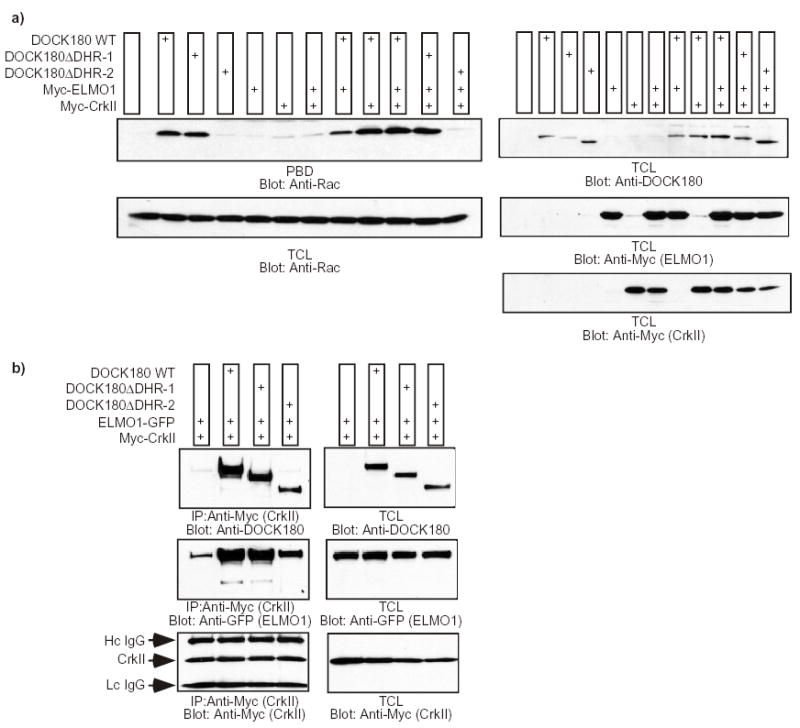
a) LR73 cells were transfected with the indicated plasmids and the GTP-loading status of Rac was analyzed by affinity precipitation with the PBD domain of PAK immobilized to glutathione-sepharose beads (upper panel). As a loading control for Rac and to verify the expression of each protein, 20 μl of total cell lysates (TCL) were analyzed by immunoblotting with antibodies against Rac, DOCK180 and Myc (for ELMO1 and CrkII). The experiment was performed independently three times, and no statistical differences were observed between the capabilities of the wild-type DOCK180 and the DOCK180ΔDHR-1 mutant to induce GTP-loading of Rac (the DOCK180ΔDHR-1 mutant demonstrated, in arbitrary units, a 0.94±0.05-fold Rac activation when co-expressed with ELMO1 and CrkII, compared to 1.0-fold activation by wild-type DOCK180, Crk and ELMO). b) DOCK180 lacking the DHR-1 forms a trimolecular complex with ELMO1 and CrkII. LR73 cells were transfected with the indicated plasmids and Triton X-100 lysates were immunoprecipitated with an antibody against the Myc-epitope. The coprecipitation of the various DOCK180 proteins and ELMO1-GFP, in addition to the verification of equal Myc-tagged CrkII precipitation, was analyzed by immunoblotting with antibodies against DOCK180 (C19), GFP and Myc, respectively (left panels). The expression levels of each protein were analyzed by immunoblotting 10 μg of the total Triton X-100 lysates with antibodies against DOCK180 (C19), GFP and Myc (right panels).
