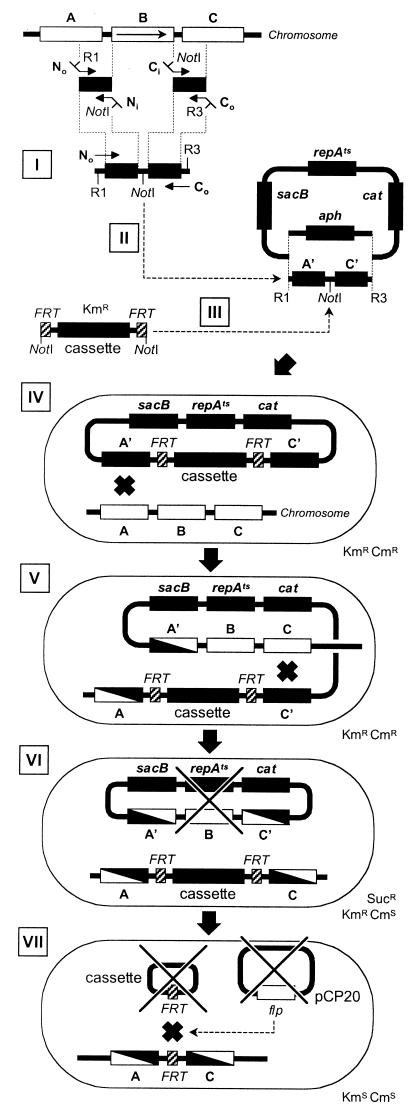
FIG.3.
Gene replacement-deletion procedure. Wild-type genes are indicated by open boxes, while engineered sequences or plasmid genes are indicated by solid boxes. B, gene targeted for deletion; A and C, genes flanking B; sacB, levan sucrase gene, toxic in the presence of sucrose (Suc); repAts, temperature-sensitive system of replication; cat and aph, genes for Cmr and Kmr, respectively. The steps of the procedure are as follows. (I) Construction of a deleted locus by crossover PCR. (II) Cloning of the crossover PCR product into pTOF24 or pTOF25 using the restriction sites (R1 and R3) provided by the outside primers (No and Co). The Kmr gene (aph) of the vector is replaced by the insert, and transformants are selected on LB-chloramphenicol medium at 30°C. (III) Cloning of an FRT cassette between the two arms of the crossover PCR insert into the unique NotI site initially provided by the internal primers Ni and Ci. Kmr is reestablished by the acquisition of the FRT cassette, and transformants are selected on LB-chloramphenicol-kanamycin plates at 30°C. (IV) First homologous recombination taking place after the introduction of the replacement plasmid in a Δlac strain. (V) Selection of integrants on LB-kanamycin-chloramphenicol plates at 42°C. (VI) Excision of the plasmid after a second homologous recombination. Allele replacement and concomitant plasmid loss (×) are selected for on LB-kanamycin-sucrose plates at 37°C. (VII) Excision of the FRT cassette after transformation with pCP20. The circular form of the cassette does not replicate and disappears, while pCP20 can be cured at 42°C. The resulting strain is Kms Cms Aps.