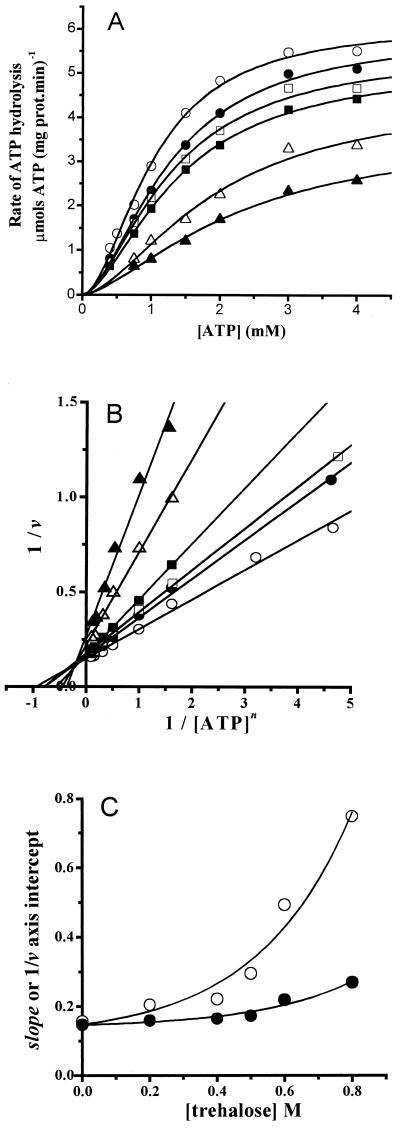
FIG. 1.
Trehalose-mediated inhibition of the plasma membrane H+-ATPase. Initial rates of ATP hydrolysis were measured at 20°C as described in Materials and Methods. The reaction mixture contained 10 mM PIPES (pH 7.0), 80 mM KCl, 5 mM sodium azide, 5 mM phosphoenolpyruvate, 200 μM NADH, 12.5 IU of pyruvate kinase, 10.45 IU of lactic dehydrogenase, 5 mM MgCl2, and the indicatedconcentrations of ATP; the final volume was 2 ml. The H+-ATPase (4.3 μg of protein in 4 μl) was added to start the reaction. (A) Plot of ATP hydrolysis versus ATP concentration at the following molar concentrations of trehalose: 0 (○), 0.2 (•), 0.4 (□), 0.5 (▪), 0.6 (▵), and 0.8 (▴). The points are the means of three independent experiments. In all cases, the standard deviation was less than 5%. The lines are the result of the best fit to the Hill equation (equation 2) by nonlinear regression. (B) Lineweaver-Burk plot of the data in panel A; the lines were obtained by linear regression of the data. (C) Replots of the slope (○) and the 1/v axis intercept (•) in panel B. The lines are the best fit to the equation y = a + b·exp(c·x), where a is the ordinate, b and c are constants, and x is the trehalose concentration.