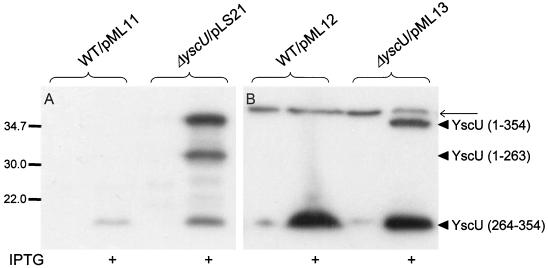
FIG. 5.
The cytoplasmic domain of YscU is proteolytically cleaved at proline-264. The cleavage pattern of full-length YscU was analyzed in the yscU-null mutant strain YPIII(pIB75) with pLS21 or pML13 expressing a wild-type or a Flag-tagged form of YscU, respectively. For analysis of the cleavage of the cytoplasmic domain (amino acids 211 to 354) of the protein, the wild-type strain YPIII(pIB102) with pML11 or pML12 was utilized, expressing a nontagged and a Flag-tagged variant of this domain of YscU, respectively. Proteins were visualized by using ECL Western blot to whole-cell fractions with antiserum raised against the cytoplasmic domain of YscU (amino acids 202 to 354) (A) or monoclonal antibodies directed against the Flag epitope (B). The different forms of YscU indicated in the figure are as follows: the full-length protein (amino acids 1 to 354), the N-terminal cleavage product (amino acids 1 to 263), and the C-terminal cleavage product (amino acids 264 to 354). The bacteria were cultured in BHI depleted for calcium at 37°C, with or without 0.4 mM IPTG, as indicated in the figure. The thinner arrow points to a nonspecific protein band recognized by the anti Flag-antibodies, which also appears in negative controls, i.e., in Yersinia strains that do not carry Flag-tagged YscU constructs. Molecular mass markers are shown to the left in kilodaltons.