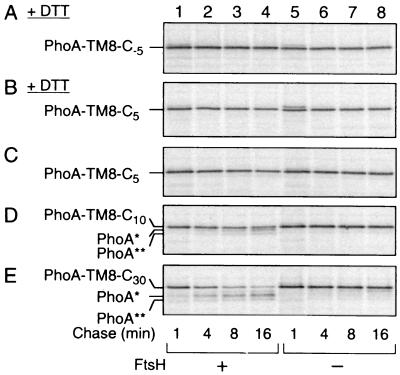
FIG. 3.
FtsH susceptibility of PhoA-TM8-Cn fusion proteins with different lengths of the cytoplasmic tail. (A and B) Resistance of PhoA-TM8-C−5 and PhoA-TM8-C5 in the absence of PhoA folding. AD1635 (ftsH+ ΔdegP; lanes 1 to 4) and AD1625 (ΔftsH ΔdegP; lanes 5 to 8) were transformed with pCH306 (PhoA-TM8-C−5) (A) or pCH306 (PhoA-TM8-C5) (B). Cells were induced for the lac transcription for 10 min and treated with 20 mM DTT for 2 min. They were then pulse-labeled with [35S]methionine for 1 min, which was followed by a chase with unlabeled methionine for 1, 4, 8, and 16 min, as indicated. (C to E) Degradation of PhoA-TM8 derivatives in the presence of PhoA folding. AR5087 (ftsH+; lanes 1 to 4) and AR5090 (ΔftsH; lanes 5 to 8) were transformed with pCH307 (carrying PhoA-TM8-C5) (C), pCH308 (carrying PhoA-TM8-C10) (D), or pCH309 (carrying PhoA-TM8-C30) (E). Cells were induced for the lac transcription for 10 min and pulse-labeled with [35S]methionine for 1 min, which was followed by a chase with unlabeled methionine for 1, 4, 8, and 16 min, as indicated. Labeled proteins (all panels) were immunoprecipitated with anti-PhoA, separated by SDS-PAGE, and visualized.