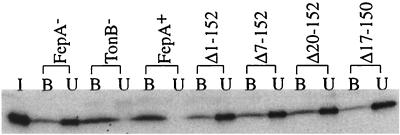
FIG. 3.
FepA globular domain deletions do not bind colicin B. KP1411 (W3110, fepA::kan, recA::cat, aroB) expressing wild-type or mutant FepA was collected at room temperature, washed once in λ-calcium buffer (10 mM Tris-HCl, pH 7.8, 20 mM MgSO4, and 5 mM CaCl2), and suspended in 0.1 volume of λ-calcium buffer. Purified colicin B (estimated at 3.5 × 1013 colicin B molecules/μl) was diluted and added to the bacteria at a ratio of 1 colicin B to 13 FepA, with FepA calculations based on recent per-cell determinations (13). After incubation at 37°C for 30 min, bound colicin B was collected by centrifugation at 16,000 × g for 5 min at 4°C. Unbound colicin B was collected from the resultant supernatants by precipitation with trichloroacetic acid. Immunoblots of samples from an entire experiment were resolved on sodium dodecyl sulfate-11% polyacrylamide gels and developed with anti-colicin B antibody at 1:5,000. I, initial amount of colicin B; U, unbound colicin B; and B, bound colicin B.