Abstract
Experiments were carried out to find if there were post-synaptic effects produced by impulses in the long ranging primary afferents, which had been shown by Wall & Werman (1976) to extend from upper lumbar dorsal roots to the sacral segments. Dorsal rootlets were stimulated in decerebrate low-spinal adult cats.
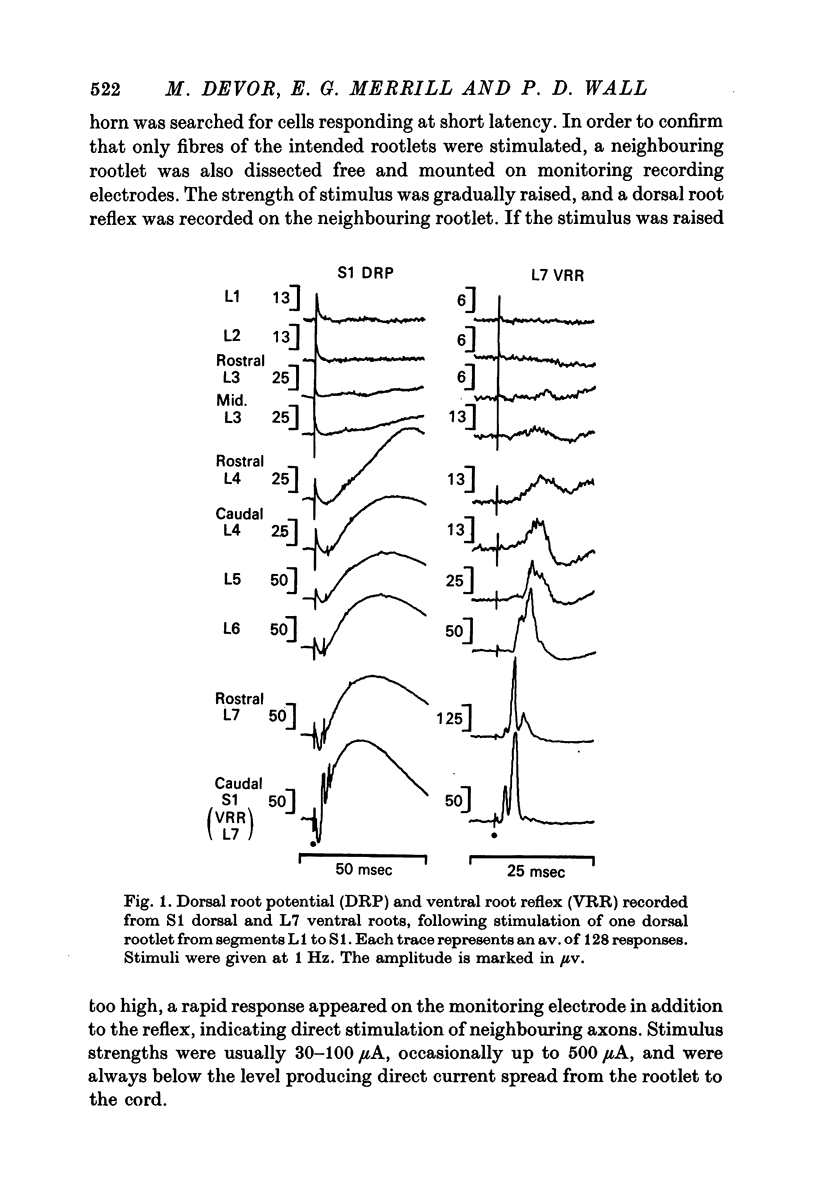
1. The dorsal root potential and ventral root reflex were recorded on S1 root filaments, in response to stimulation of dorsal rootlets extending from L1 to S1. With increasing distance between stimulating and recording segments, these potentials became smaller and more delayed. In two animals, there was no response at S1 to stimulation of L1 and L2 dorsal roots.
2. In all animals, stimulation of L3 or L4 dorsal roots produced cell responses in dorsal horn segments L7 or S1. The density of such cells was variable, from animal to animal. Responding cells were mainly concentrated laterally in the dorsal horn.
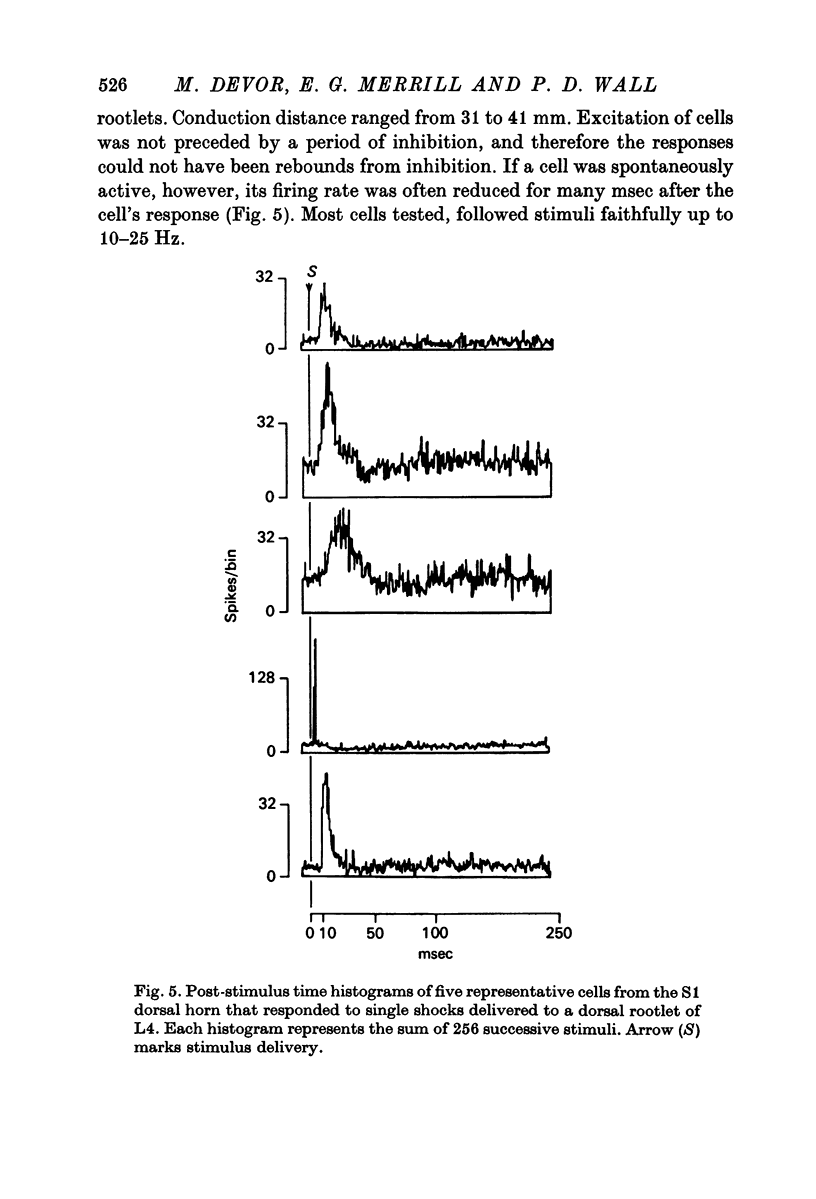
3. The latency and response variability of L7-S1, dorsal horn cells to L3-L4 stimulation was consistent with at least some of them being fired monosynaptically.
4. Cells that respond to stimulation of one distant rootlet respond to many closer rootlets as well.
5. The receptive fields of L7-S1 dorsal horn cells, responsive to stimulation of L3-L4 rootlets, were typical of those generally found in the L7-S1 segments, and were at some distance from the L4 dermatome. Only twenty cells had receptive fields which extended into the dermatome of the rootlets stimulated.
6. It was established that some L4 cells respond to S1 dorsal root stimulation, just as the main study had shown that S1 responds to L4.
7. It is concluded that substantial numbers of dorsal horn cells, including cells with many types of cutaneous receptive field, respond to two classes of synaptic in-put: one effective in firing the cell upon natural cutaneous stimulation, and one relatively ineffective, capable of driving the cell only when stimulated electrically and thus carrying a synchronous volley from a number of highly convergent axons. The contribution of this secondary afferent channel to normal and pathological cord physiology has now to be determined.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum A. I., Wall P. D. Chronic changes in the response of cells in adult cat dorsal horn following partial deafferentation: the appearance of responding cells in a previously non-responsive region. Brain Res. 1976 Nov 5;116(2):181–204. doi: 10.1016/0006-8993(76)90899-4. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Brown P. B., Fuchs J. L. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975 Jan;38(1):1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Coulter J. D., Willis W. D. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Exp Brain Res. 1973 Apr 30;17(2):177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Dorsal horn cells with proximal cutaneous receptive fields. Brain Res. 1976 Dec 17;118(2):325–328. doi: 10.1016/0006-8993(76)90719-8. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kusama T. Distribution of the dorsal root fibers in the cat. An experimental study with the Nauta method. Brain Res. 1969 Apr;13(2):338–359. doi: 10.1016/0006-8993(69)90292-3. [DOI] [PubMed] [Google Scholar]
- KUHN R. A. Organization of tactile dermatomes in cat and monkey. J Neurophysiol. 1953 Mar;16(2):169–182. doi: 10.1152/jn.1953.16.2.169. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C. Electrotonus in dorsal nerve roots. Cold Spring Harb Symp Quant Biol. 1952;17:203–219. doi: 10.1101/sqb.1952.017.01.020. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Wall P. D. Factors forming the edge of a receptive field: the presence of relatively ineffective afferent terminals. J Physiol. 1972 Nov;226(3):825–846. doi: 10.1113/jphysiol.1972.sp010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réthelyi M., Szentágothai J. The large synaptic complexes of the substantia gelatinosa. Exp Brain Res. 1969;7(3):258–274. doi: 10.1007/BF00239033. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M., HONGCHIEN H. A. THE TERMINAL FIELDS OF DORSAL ROOT FIBERS IN THE LUMBOSACRAL SPINAL CORD OF THE CAT, AND THE DENDRITIC ORGANIZATION OF THE MOTOR NUCLEI. Prog Brain Res. 1964;11:120–154. doi: 10.1016/s0079-6123(08)64046-7. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal patterns in cat spinal cord. 3. Primary afferent collaterals. Brain Res. 1969 May;13(3):417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D. The presence of ineffective synapses and the circumstances which unmask them. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Werman R. The physiology and anatomy of long ranging afferent fibres within the spinal cord. J Physiol. 1976 Feb;255(2):321–334. doi: 10.1113/jphysiol.1976.sp011282. [DOI] [PMC free article] [PubMed] [Google Scholar]


