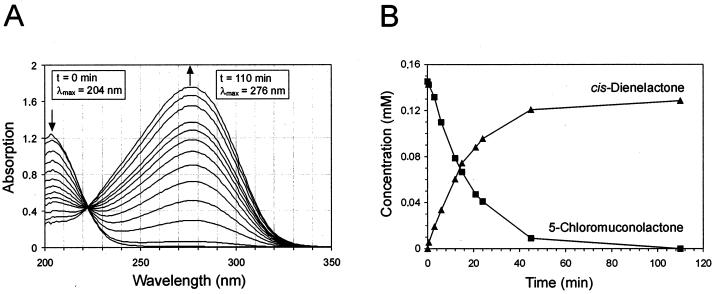
FIG. 7.
(A) Spectral changes during the ClcF-catalyzed conversion of 5-chloromuconolactone to cis-dienelactone. (B) Stoichiometry of the reaction, estimated by reversed-phase HPLC. A 0.2-μmol amount of 5-chloromuconolactone was incubated with 0.01 μg of purified ClcF (7 × 10−3 U as measured with 5-chloromuconolactone) in phosphate buffer (pH 7.2) at room temperature. UV spectra were recorded at 0, 0.5, 3, 6, 9, 12, 15, 18, 21, 24, 45, and 110 min. From an identical second reaction mixture, samples were taken at similar intervals. After quenching by addition of 10% (vol/vol) ortho-phosphoric acid, those samples were subjected to HPLC.