Abstract
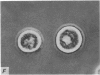
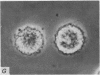
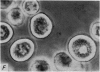
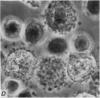
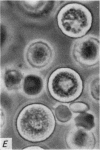
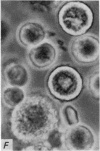
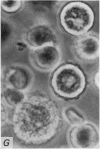
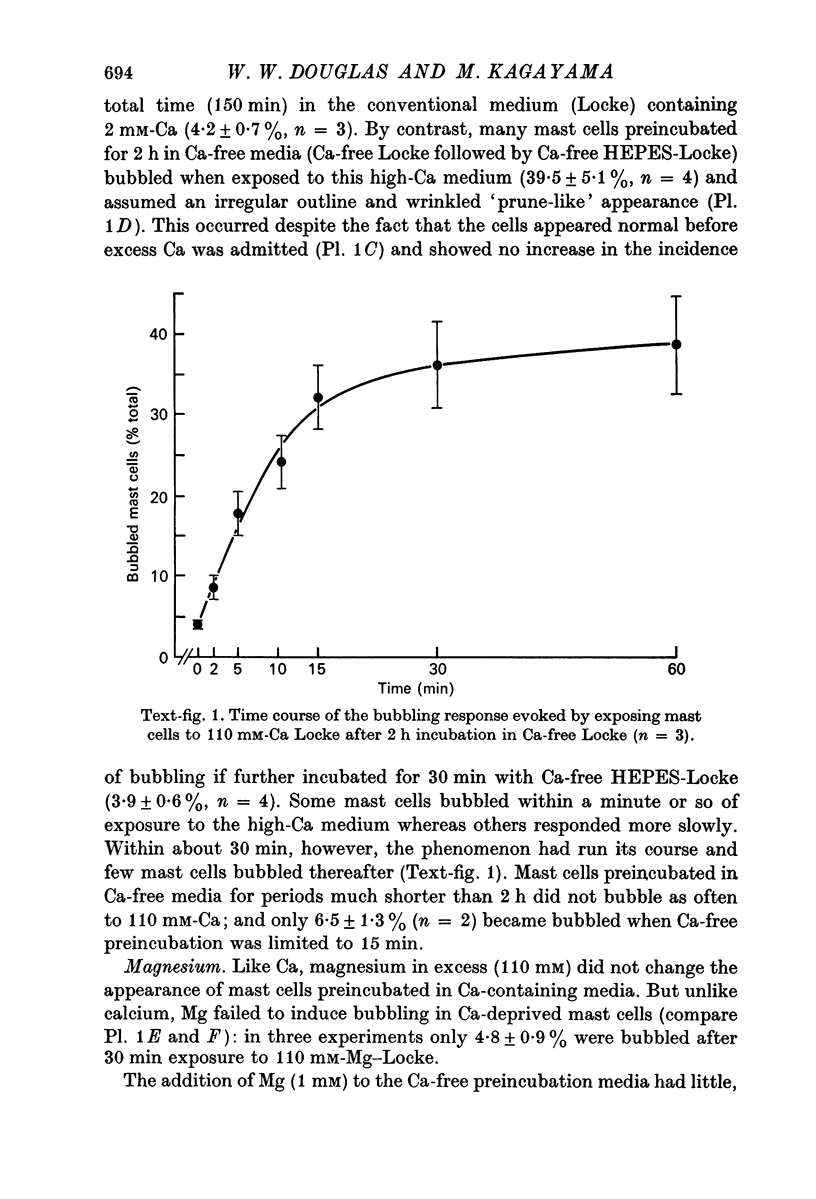
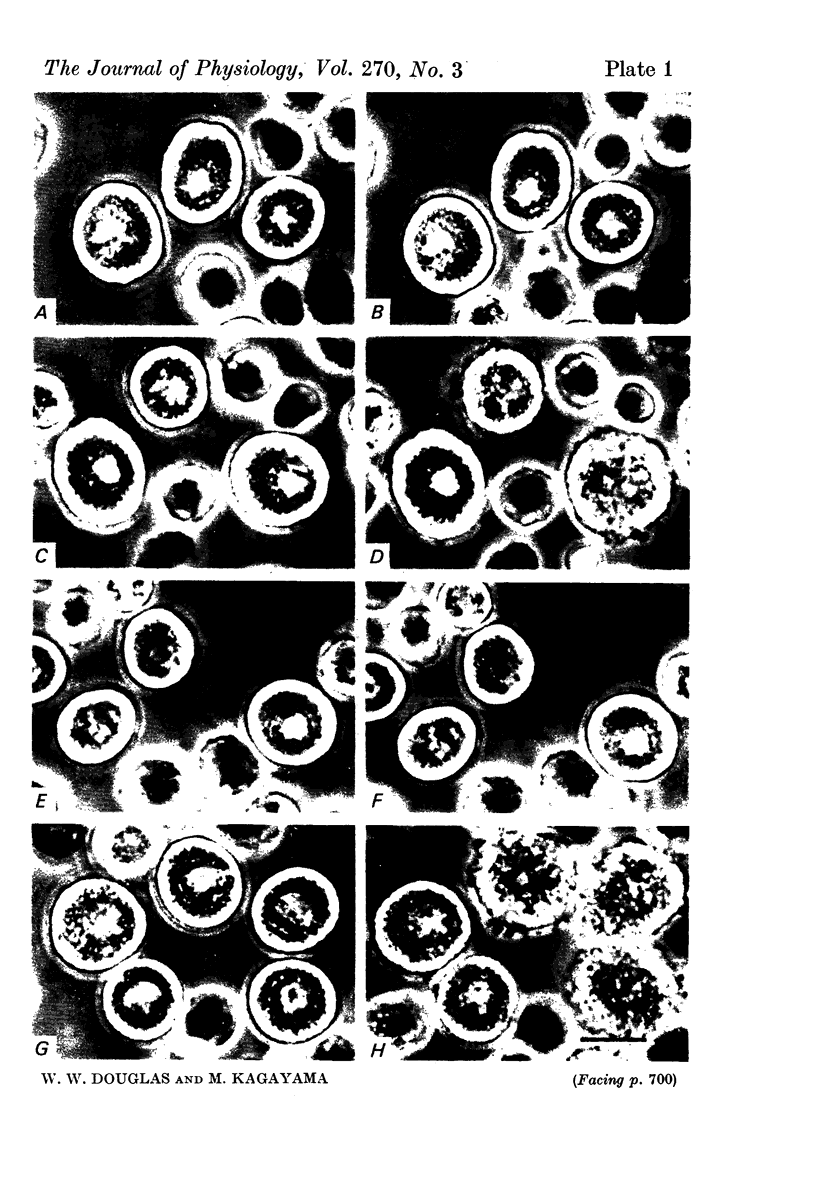
1. Isolated rat peritoneal mast cells incubated in Ca-free media for 2 h, with or without EDTA, and observed by phase-contact microscopy, became `bubbled' in appearance when subsequently exposed to media rich in calcium (16-110 mM).
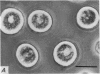
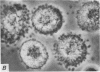
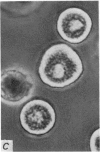
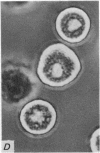
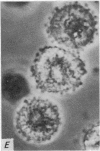
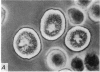
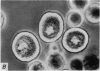
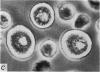
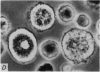
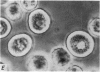
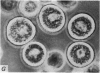
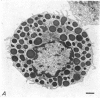
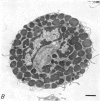
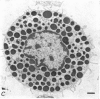
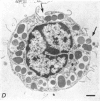
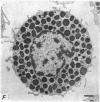
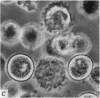
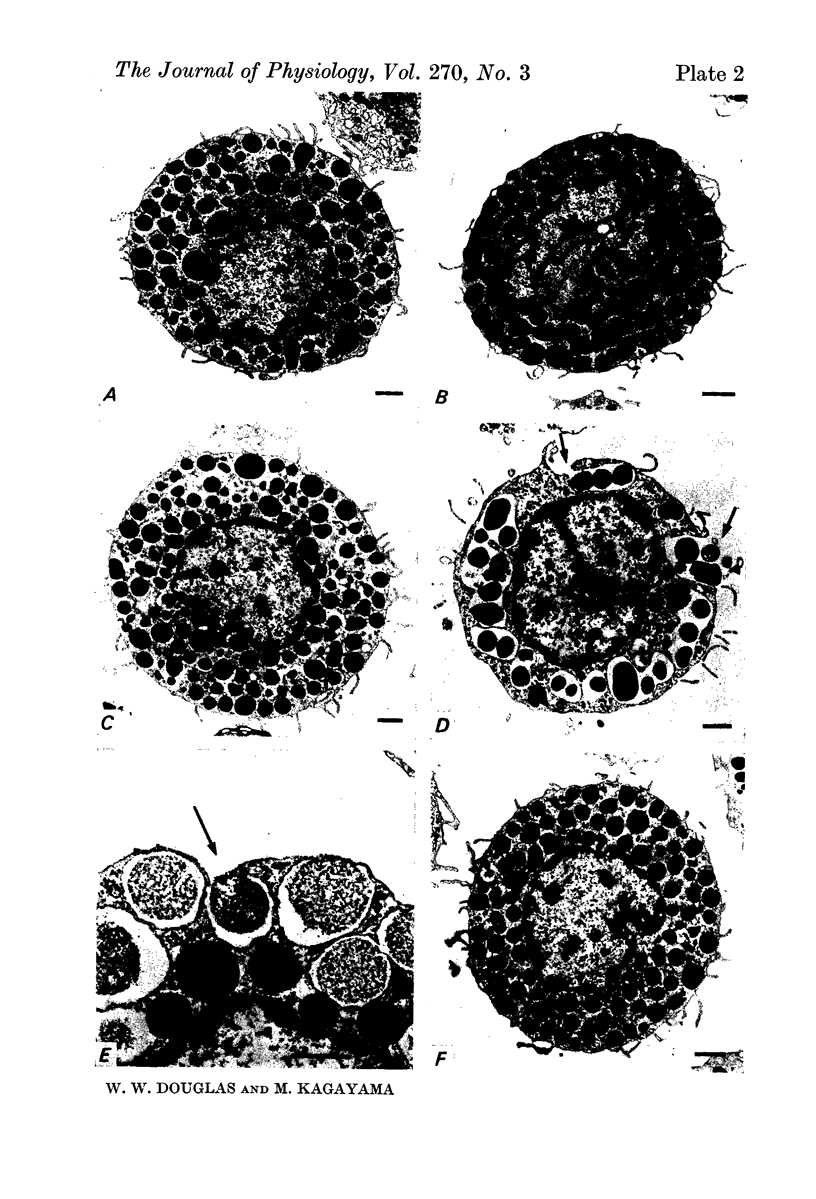
2. Electron microscopy showed the response to be `compound' exocytosis of the sort elicited by conventional mast cell secretagogues such as antigen (in sensitized cells) and 48/80.
3. The response to Ca was inhibited by withdrawing glucose and adding dinitrophenol and was thus energy-dependent.
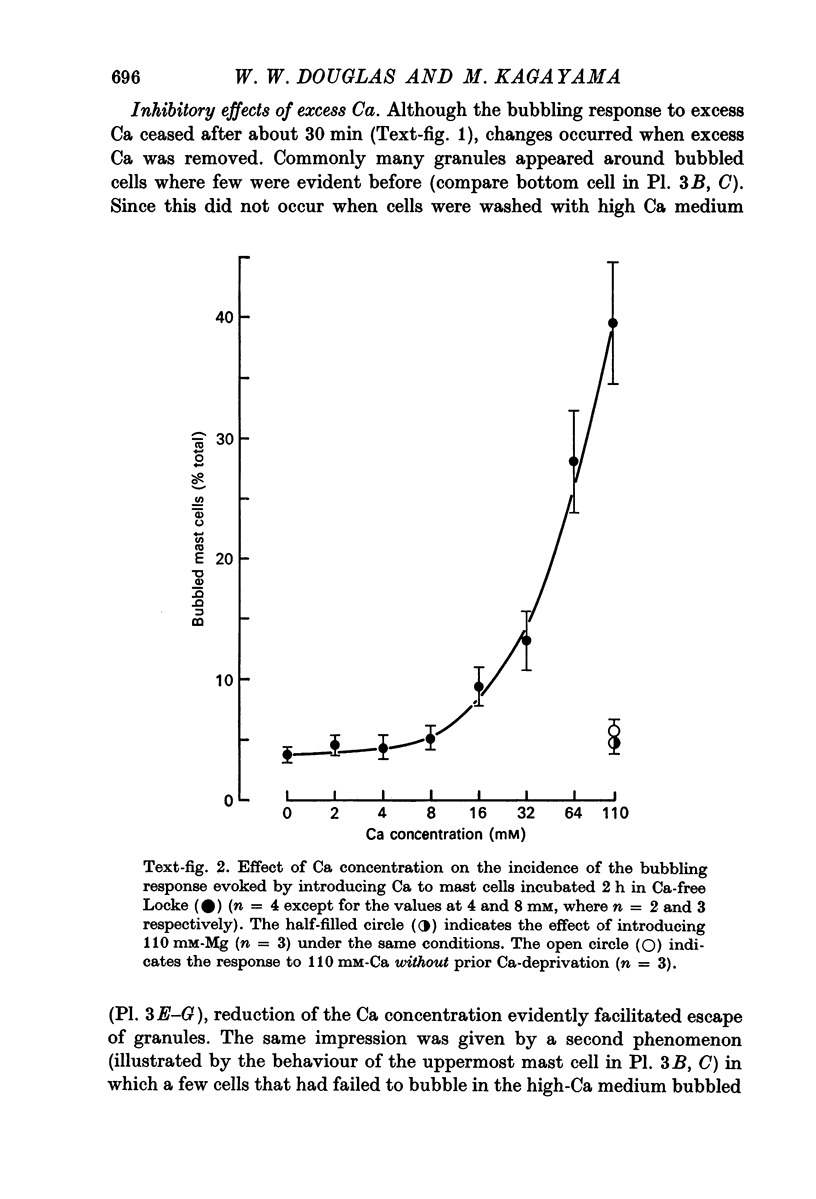
4. Mg in similarly high concentration had no such stimulant effect on Ca-deprived cells, and excess Ca stimulated only after Ca deprivation.
5. It is suggested that Ca deprivation may increase the permeability of the plasma membrane of the mast cell thereby allowing some Ca, when subsequently introduced in high concentration, to penetrate and activate exocytosis; and the results are considered further support for the postulated mediator function of Ca in stimulus-secretion coupling.
6. Two inhibitory effects of calcium in high concentration were detected: (a) suppression of migration or expulsion of granules from the exocytotic pits within the cellular domain; and (b) diminished sensitivity to 48/80.
Full text
PDF







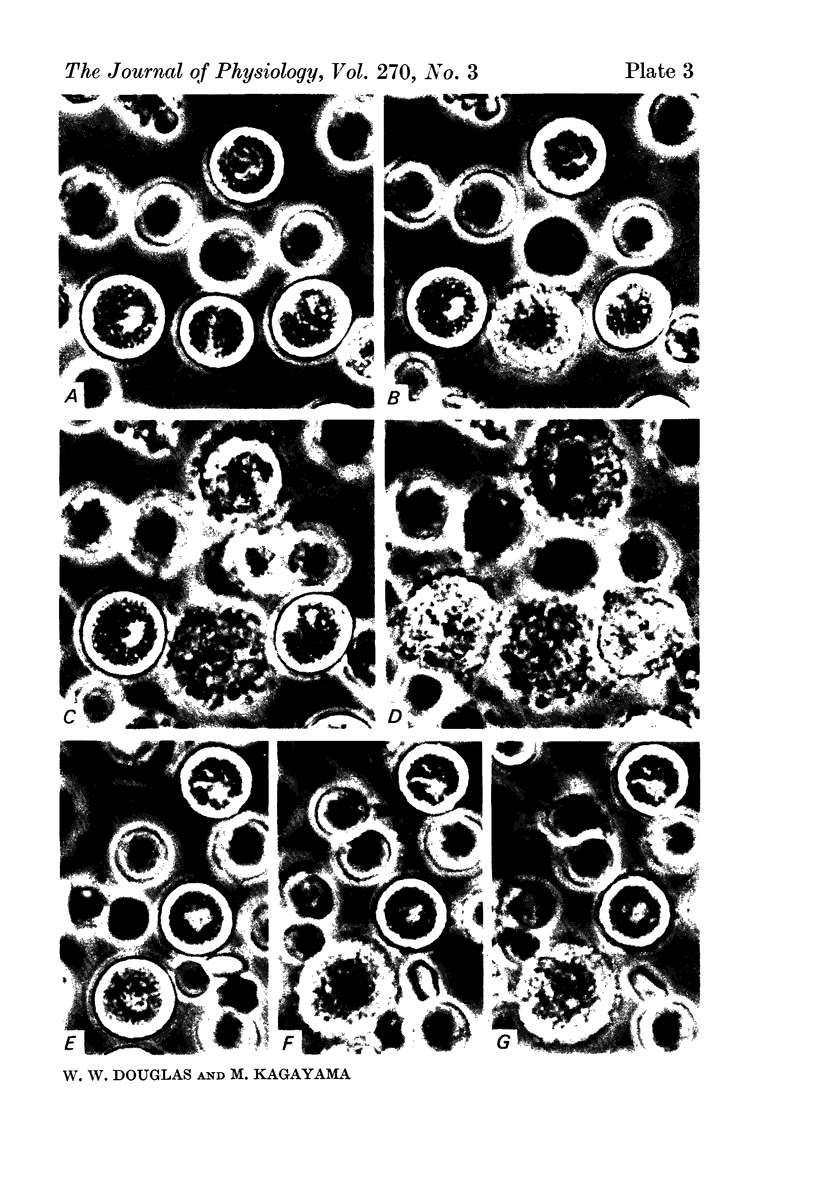
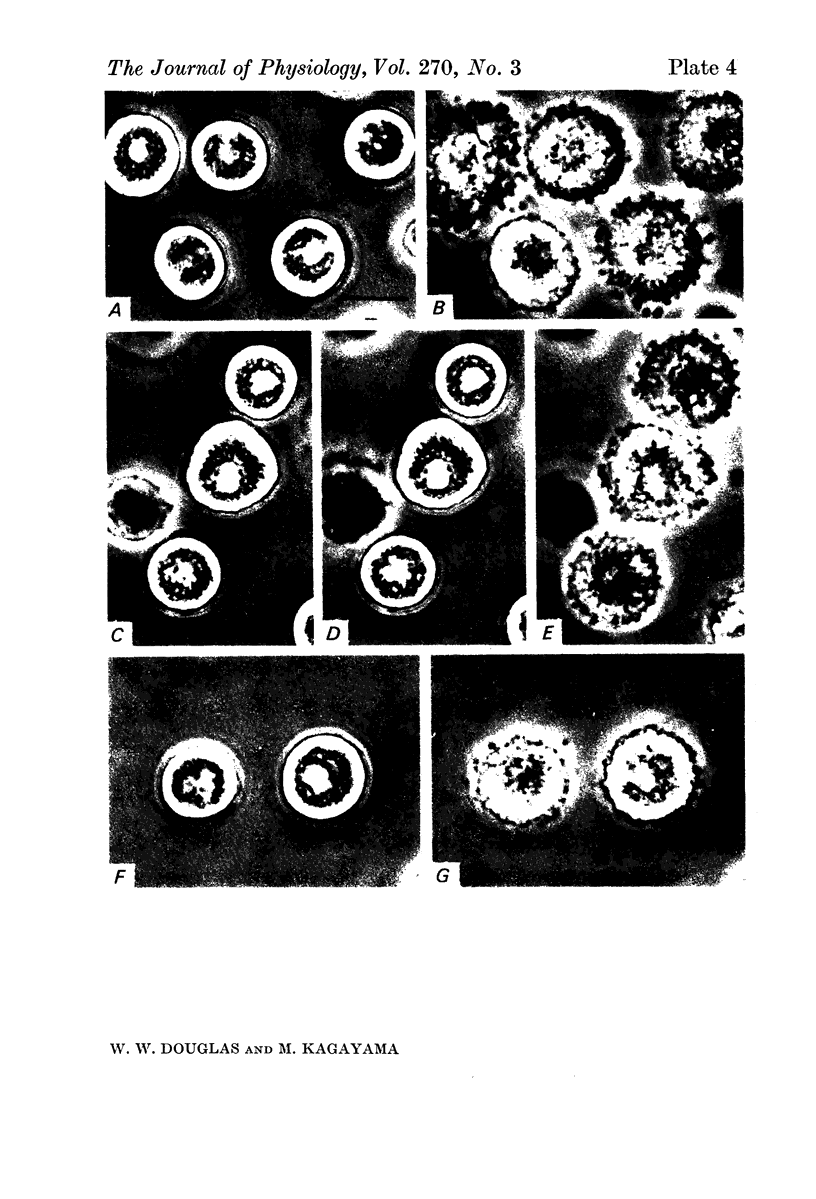



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Slorach S. A., Uvnäs B. Sequential exocytosis of storage granules during antigen-induced histamine release from sensitized rat mast cells in vitro. An electron microscopic study. Acta Physiol Scand. 1973 Jul;88(3):359–372. doi: 10.1111/j.1748-1716.1973.tb05465.x. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Histamine release by exocytosis from rat mast cells on reduction of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or magnesium. J Physiol. 1976 May;257(2):433–448. doi: 10.1113/jphysiol.1976.sp011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. Stimulation of uptake of calcium-45 in the adrenal gland by acetylcholine. Nature. 1961 Dec 30;192:1299–1299. doi: 10.1038/1921299a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B. Energy production in rat mast cells and its role for histamine release. Int Arch Allergy Appl Immunol. 1975;49(1-2):155–171. doi: 10.1159/000231391. [DOI] [PubMed] [Google Scholar]
- Diamant B., Patkar S. A. Stimulation and inhibition of histamine release from isolated rat mast cells. Dual effects of the ionophore A23187. Int Arch Allergy Appl Immunol. 1975;49(1-2):183–207. doi: 10.1159/000231394. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Ueda Y. Proceedings: Mast cell secretion (histamine release) induced by 48-80: calcium-dependent exocytosis inhibited strongly by cytochalasin only when glycolysis is rate-limiting. J Physiol. 1973 Oct;234(2):97P–98P. [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield G. I. The effect of compound 48/80 on the rat mast cell. J Pathol Bacteriol. 1965 Oct;90(2):599–605. doi: 10.1002/path.1700900228. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Douglas W. W. Electron microscope evidence of calcium-induced exocytosis in mast cells treated with 48-80 or the ionophores A-23187 and X-537A. J Cell Biol. 1974 Aug;62(2):519–526. doi: 10.1083/jcb.62.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. The mechanism of histamine release from mast cells. Biochem Pharmacol. 1972 Jul 15;21(14):1889–1896. doi: 10.1016/0006-2952(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr T. S., Hall D. E., Allison A. C. Role of contractile microfilaments in the release of histamine from mast cells. Nature. 1972 Apr 14;236(5346):350–351. doi: 10.1038/236350a0. [DOI] [PubMed] [Google Scholar]
- Peterson C. Role of energy metabolism in histamine release. A study on isolated rat mast cells. Acta Physiol Scand Suppl. 1974;413:1–34. [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P. Membrane-associated actin filaments in the cortical cytoplasm of the rat mast cell. Exp Cell Res. 1975 Jul;93(2):293–298. doi: 10.1016/0014-4827(75)90453-x. [DOI] [PubMed] [Google Scholar]
- UVNAES B. RELEASE PROCESSES IN MAST CELLS AND THEIR ACTIVATION BY INJURY. Ann N Y Acad Sci. 1964 Aug 27;116:880–890. doi: 10.1111/j.1749-6632.1964.tb52554.x. [DOI] [PubMed] [Google Scholar]