Abstract
1. Habituation of the lateral giant fibre escape response in the crayfish to repetitive tactile stimuli is believed to result from homosynaptic depression at the first synapse of the reflex, between tactile afferents and interneurones. Normally, habituation of escape responses to repeated innocuous stimuli is presumed to be adaptive. Experiments reported here were undertaken to determine whether habituation would occur under circumstances when it would presumably be maladaptive — in particular, when tactile receptors are stimulated by an animal's own tail-flip movements.
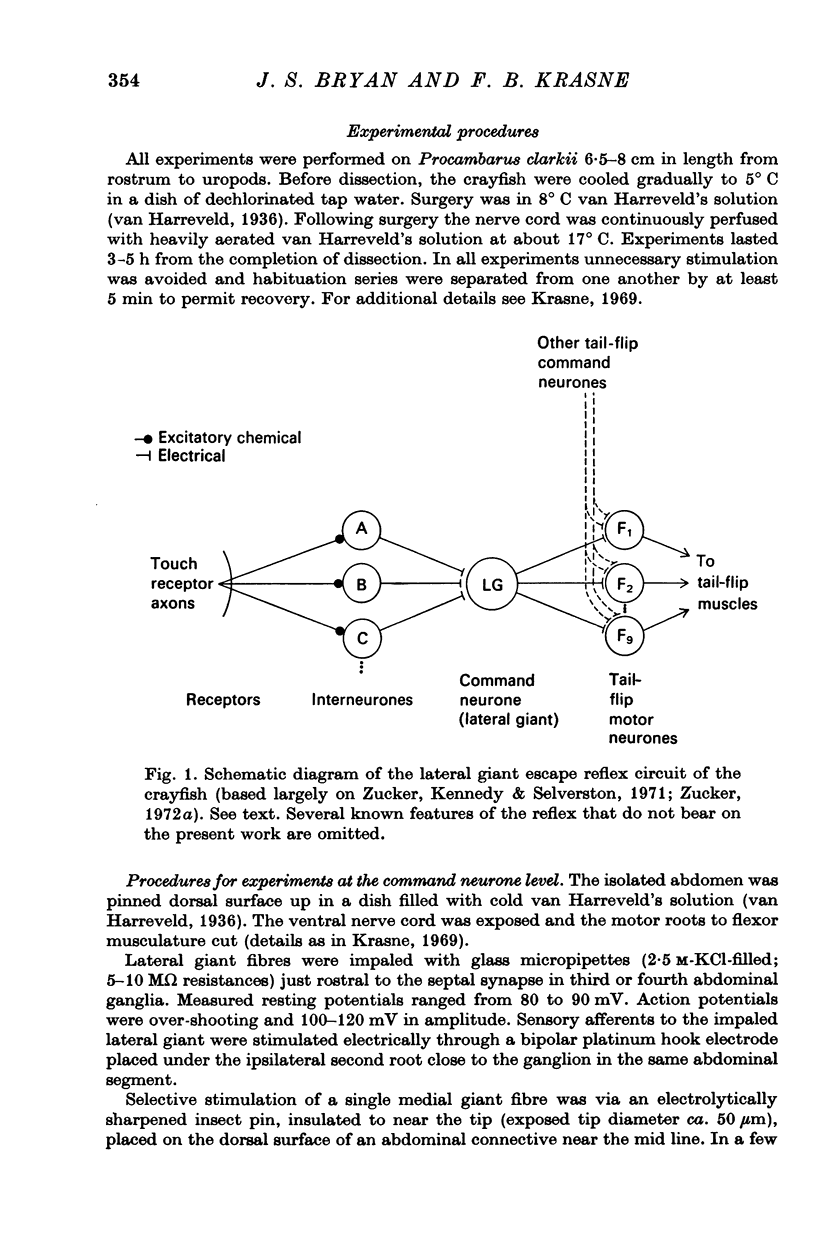
2. Experiments were carried out on the crayfish isolated abdominal nerve cord, which contains the lateral giant reflex pathway.
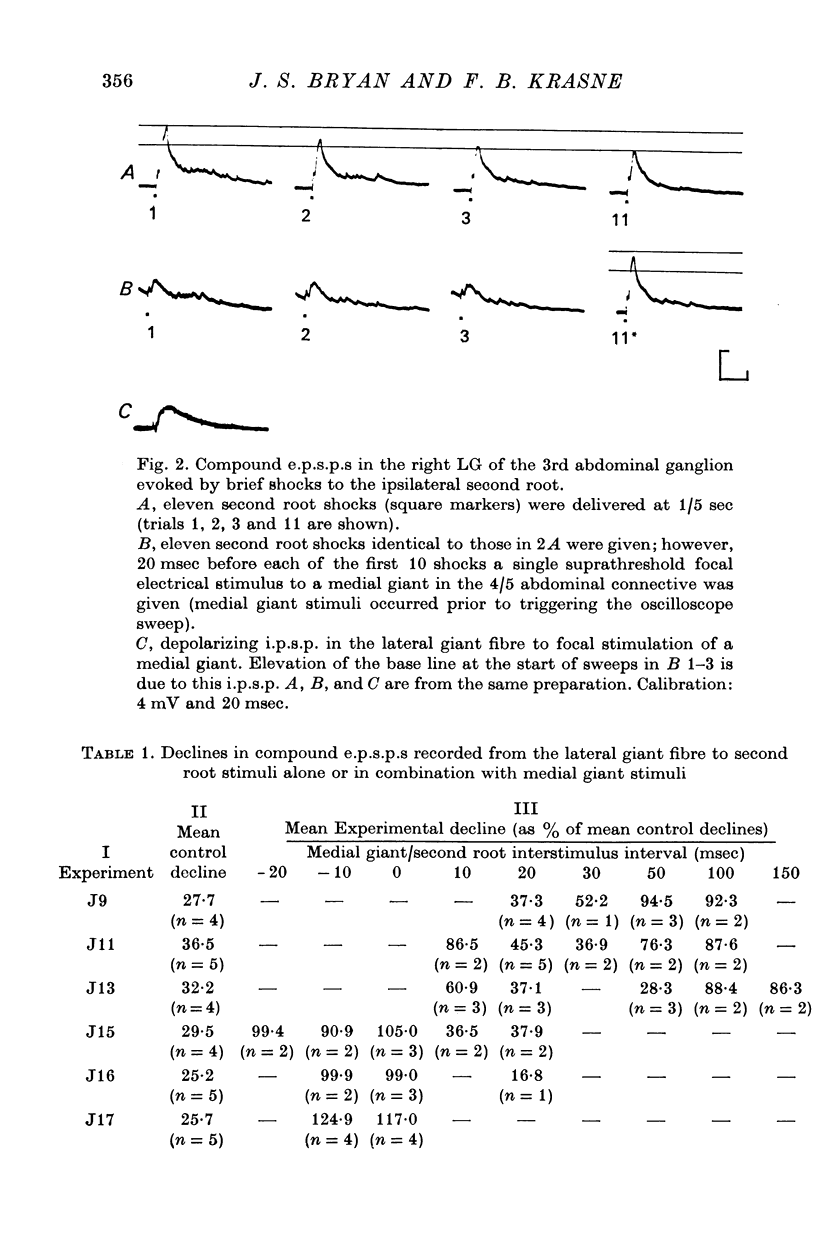
3. Compound e.p.s.p.s elicited in the lateral giant by electrical stimulation of tactile afferents decline by from 25 to 36% over a series of eleven trials at 1/5 sec (control series).
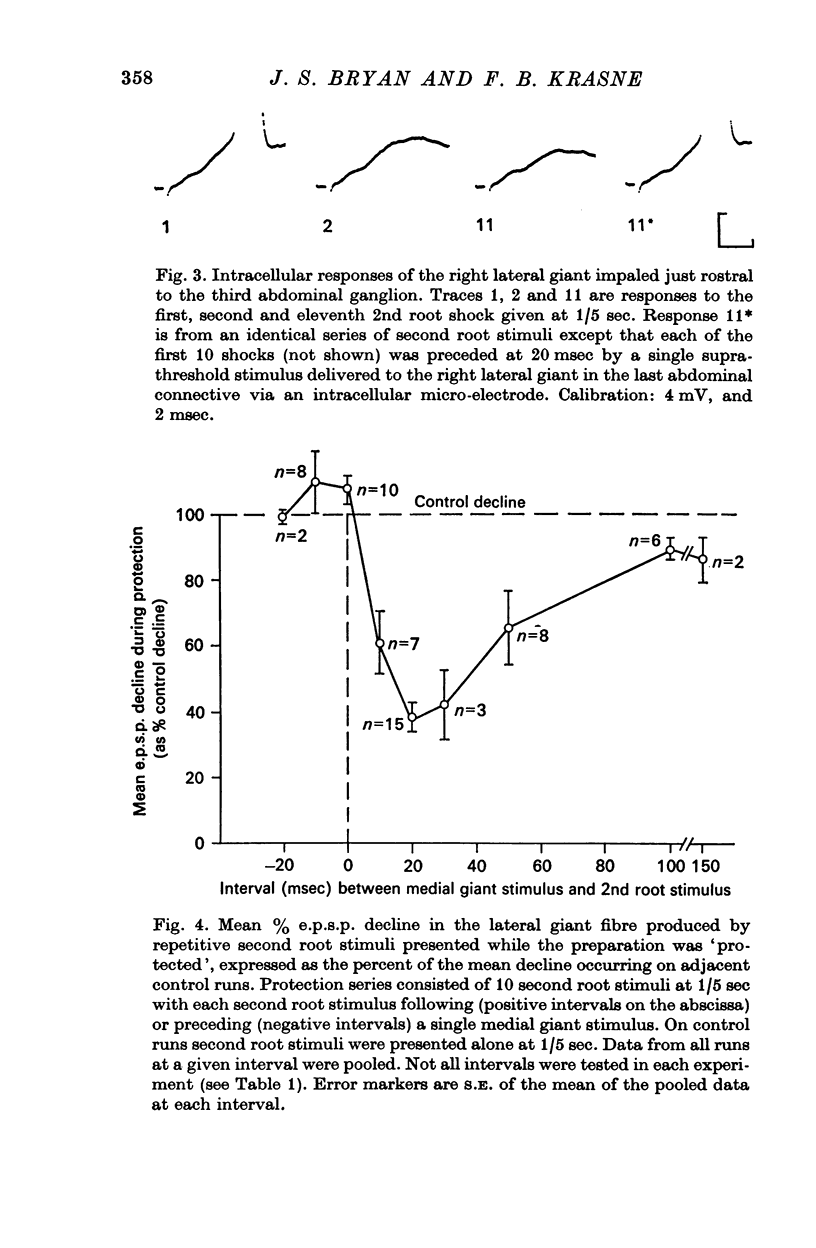
4. To determine whether such a decline would occur when sensory afferents are stimulated during a `tail-flip', stimuli were given as in the control series but each stimulus occurred 20 msec after direct electrical stimulation of a medial giant or lateral giant escape-command fibre at which time tail flexion movements of an intact animal would be in progress. Under these conditions% e.p.s.p. decline over 11 trials at 1/5 sec was only 16-45% of that occurring on the control series.
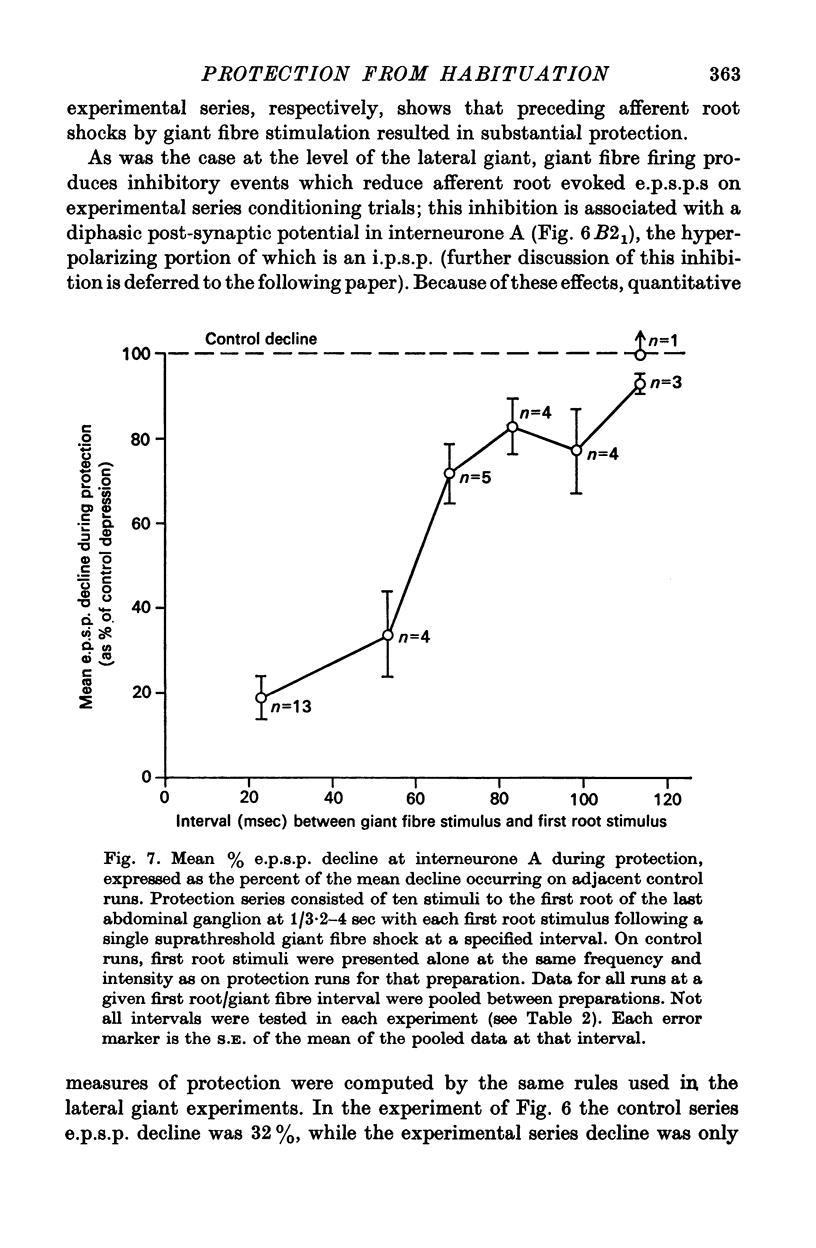
5. This protective effect starts at about 10 msec after escape command neurone firing, is maximal at 20 msec, and thereafter declines, remaining weakly detectable at 100 msec. This time course is commensurate with that required for execution of a tail-flip movement. Thus, sensory afferent-to-lateral giant transmission is protected from depression if stimuli occur when a tail-flip movement is or should be occurring.
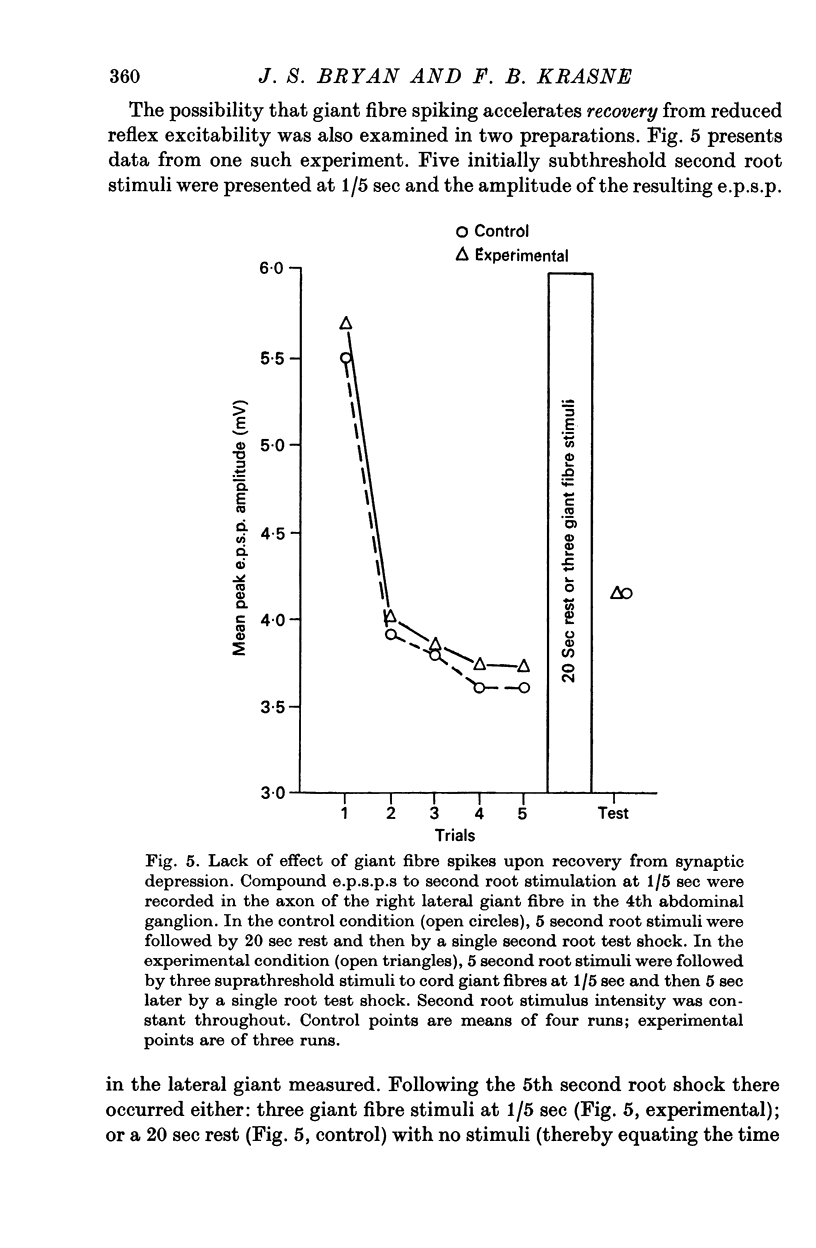
6. Giant fibre spikes do not superimpose facilitation upon a depressed reflex pathway, nor accelerate rate of recovery from depression; rather, protection is attributable to actual prevention of development of the depressed state.
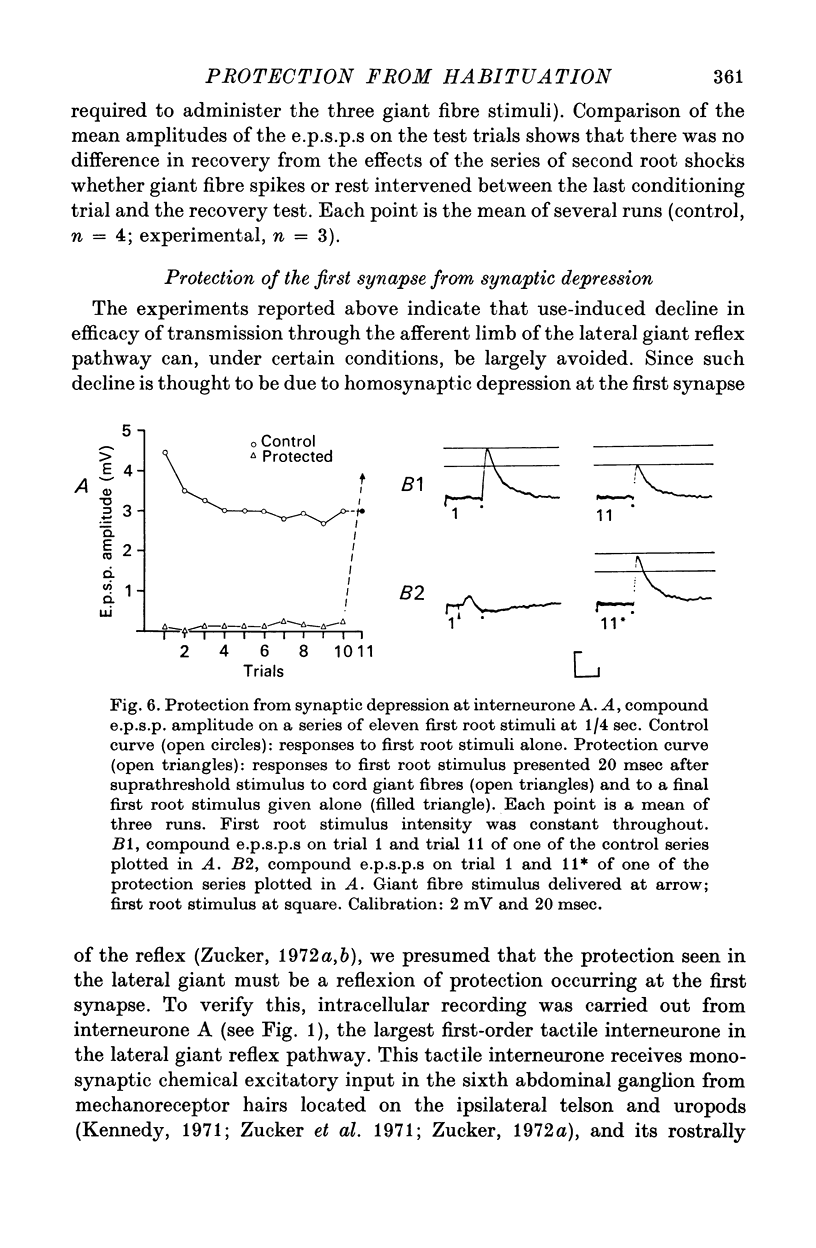
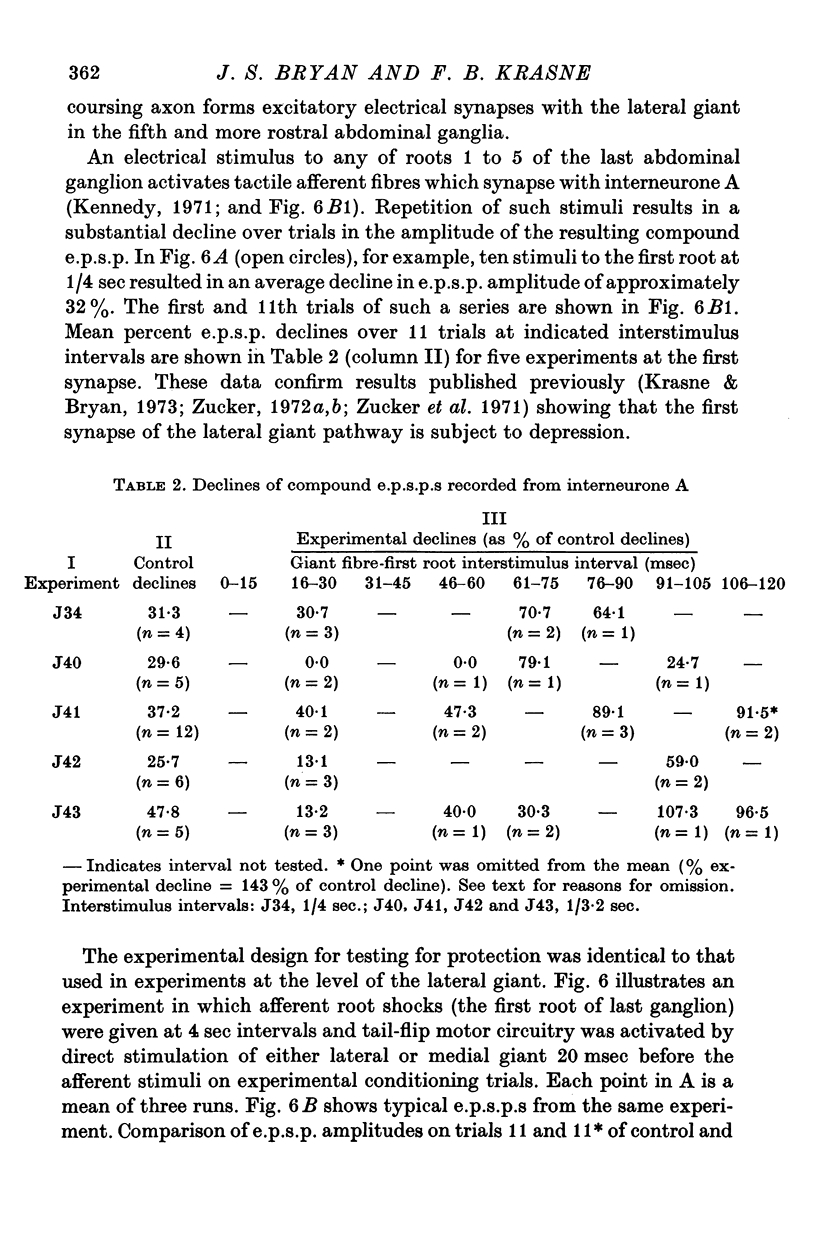
7. Protection was also examined at the first synapse of the reflex, where the depression responsible for habituation is believed to occur, by recording intracellularly in the largest of the first-order interneurones (interneurone A) of the pathway. In absence of protection, ten stimuli presented at 1/4 sec caused a mean decline of 32% in the e.p.s.p. in interneurone A. When such stimuli followed directly evoked escape command neurone firing by 20 msec this decline was reduced by 59-100%.
8. We suggest that protection serves to prevent crayfish from habituating to stimuli produced by their own tail-flip movements.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L., Bittner G. D. Matching of excitatory and inhibitory inputs to crustacean muscle fibers. J Neurophysiol. 1971 Jan;34(1):157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- Atwood H. L., Wiersma C. A. Command interneurons in the crayfish central nervous system. J Exp Biol. 1967 Apr;46(2):249–261. doi: 10.1242/jeb.46.2.249. [DOI] [PubMed] [Google Scholar]
- Benguerel A. P., McBay H. D. Changes in TTS associated with humming and nonvocal activities. J Acoust Soc Am. 1972 Oct;52(4):1107–1114. doi: 10.1121/1.1913221. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Castellucci V. F., Kandel E. R. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971 Aug;2(2):79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. Crayfish interneurons. Physiologist. 1971 Feb;14(1):5–30. [PubMed] [Google Scholar]
- Krasne F. B., Bryan J. S. Habituation: regulation through presynaptic inhibition. Science. 1973 Nov 9;182(4112):590–592. doi: 10.1126/science.182.4112.590. [DOI] [PubMed] [Google Scholar]
- Krasne F. B. Excitation and habituation of the crayfish escape reflex: the depolarizing response in lateral giant fibres of the isolated abdomen. J Exp Biol. 1969 Feb;50(1):29–46. doi: 10.1242/jeb.50.1.29. [DOI] [PubMed] [Google Scholar]
- Larimer J. L., Eggleston A. C., Masukawa L. M., Kennedy D. The different connections and motor outputs of lateral and medial giant fibres in the crayfish. J Exp Biol. 1971 Apr;54(2):391–402. doi: 10.1242/jeb.54.2.391. [DOI] [PubMed] [Google Scholar]
- Mittenthal J. E., Wine J. J. Connectivity patterns of crayfish giant interneurons: visualization of synaptic regions with cobalt dye. Science. 1973 Jan 12;179(4069):182–184. doi: 10.1126/science.179.4069.182. [DOI] [PubMed] [Google Scholar]
- O'Shea M., Rowell C. H. Protection from habituation by lateral inhibition. Nature. 1975 Mar 6;254(5495):53–55. doi: 10.1038/254053a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. Recurrent inhibition in the giant-fibre system of the crayfish and its effect on the excitability of the escape response. J Exp Biol. 1968 Jun;48(3):545–567. doi: 10.1242/jeb.48.3.545. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Remler M. P. Neural geometry and activation of crayfish fast flexor motoneurons. J Neurophysiol. 1972 Nov;35(6):797–814. doi: 10.1152/jn.1972.35.6.797. [DOI] [PubMed] [Google Scholar]
- Sherman R. G., Atwood H. L. Synaptic facilitation: long-term neuromuscular facilitation in crustaceans. Science. 1971 Mar 26;171(3977):1248–1250. doi: 10.1126/science.171.3977.1248. [DOI] [PubMed] [Google Scholar]
- TAKEDA K., KENNEDY D. SOMA POTENTIALS AND MODES OF ACTIVATION OF CRAYFISH MOTONEURONS. J Cell Physiol. 1964 Oct;64:165–181. doi: 10.1002/jcp.1030640203. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Spencer W. A. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966 Jan;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Woodson P. B., Schlapfer W. T., Barondes S. H. Dopamine, serotonin and related compounds: presynaptic effects on synaptic depression, frequency facilitation, and post-tetanic potentiation at a synapse in Aplysia californica. Brain Res. 1976 Jun 4;109(1):61–81. doi: 10.1016/0006-8993(76)90380-2. [DOI] [PubMed] [Google Scholar]
- WIERSMA C. A., HUGHES G. M. On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii (Girard). J Comp Neurol. 1961 Apr;116:209–228. doi: 10.1002/cne.901160209. [DOI] [PubMed] [Google Scholar]
- Wiersma C. A., Yamaguchi T. Integration of visual stimuli by the crayfish central nervous system. J Exp Biol. 1967 Dec;47(3):409–431. doi: 10.1242/jeb.47.3.409. [DOI] [PubMed] [Google Scholar]
- Wine J. J., Krasne F. B., Chen L. Habituation and inhibition of the crayfish lateral giant fibre escape response. J Exp Biol. 1975 Jun;62(3):771–782. doi: 10.1242/jeb.62.3.771. [DOI] [PubMed] [Google Scholar]
- Woodson P. B., Tremblay J. P., Schlapfer W. T., Barondes S. H. Heterosynaptic inhibition modifies the presynaptic plasticities of the transmission process at the synapse in Aplysia californica. Brain Res. 1976 Jun 4;109(1):83–95. doi: 10.1016/0006-8993(76)90381-4. [DOI] [PubMed] [Google Scholar]
- Zilber-Gachelin N. F., Chartier M. P. Modification of the motor reflex responses due to repetition of the peripheral stimulus in the cockroach. I. Habituation at the level of an isolated abdominal ganglion. J Exp Biol. 1973 Oct;59(2):359–381. doi: 10.1242/jeb.59.2.359. [DOI] [PubMed] [Google Scholar]
- Zilber-Gachelin N. F., Chartier M. P. Modification of the motor reflex responses due to repetition of the peripheral stimulus in the cockroach. II. Conditions of activation of the motoneurones. J Exp Biol. 1973 Oct;59(2):383–403. doi: 10.1242/jeb.59.2.383. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. 3. Electrical junctions and dendrite spikes in fast flexor motoneurons. J Neurophysiol. 1972 Sep;35(5):638–651. doi: 10.1152/jn.1972.35.5.638. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. I. Neural circuit exciting lateral giant fiber. J Neurophysiol. 1972 Sep;35(5):599–620. doi: 10.1152/jn.1972.35.5.599. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Kennedy D., Selverston A. I. Neuronal circuit mediating escape responses in crayfish. Science. 1971 Aug 13;173(3997):645–650. doi: 10.1126/science.173.3997.645. [DOI] [PubMed] [Google Scholar]


