Abstract
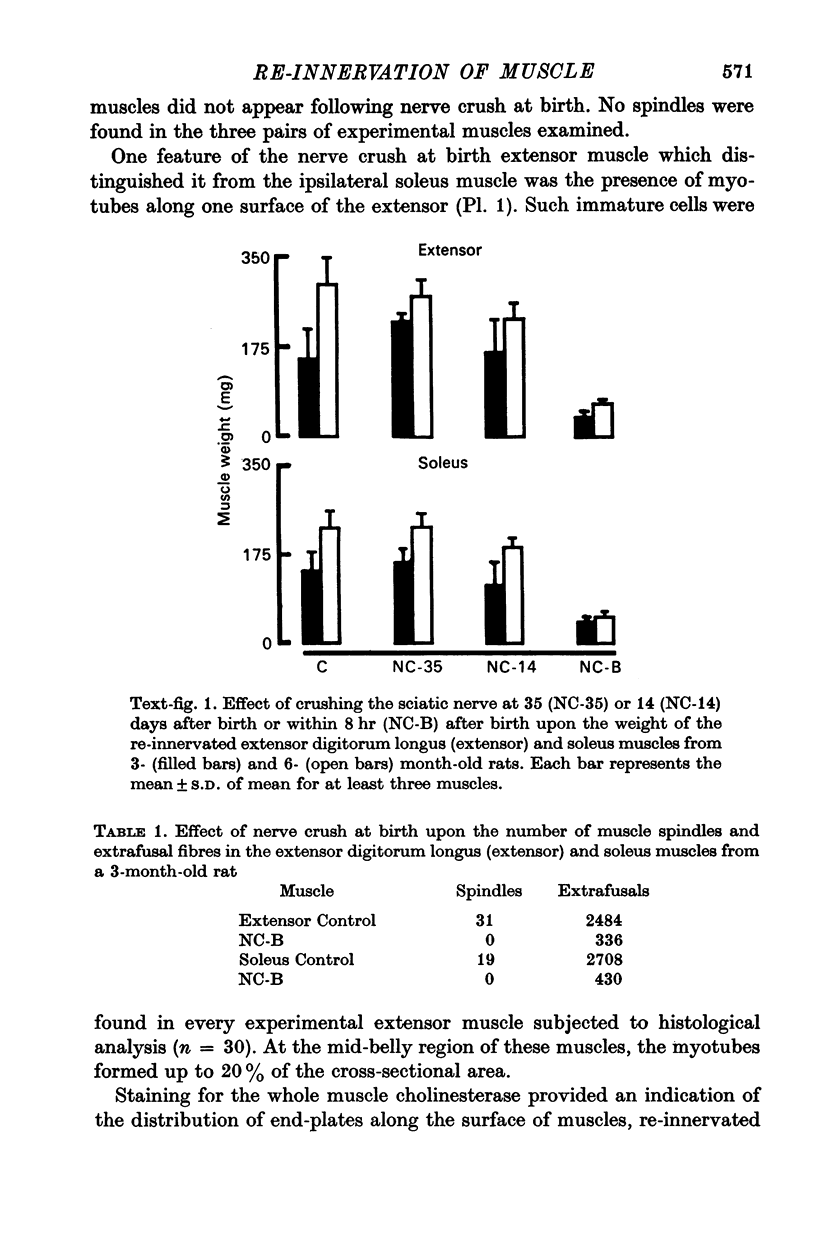
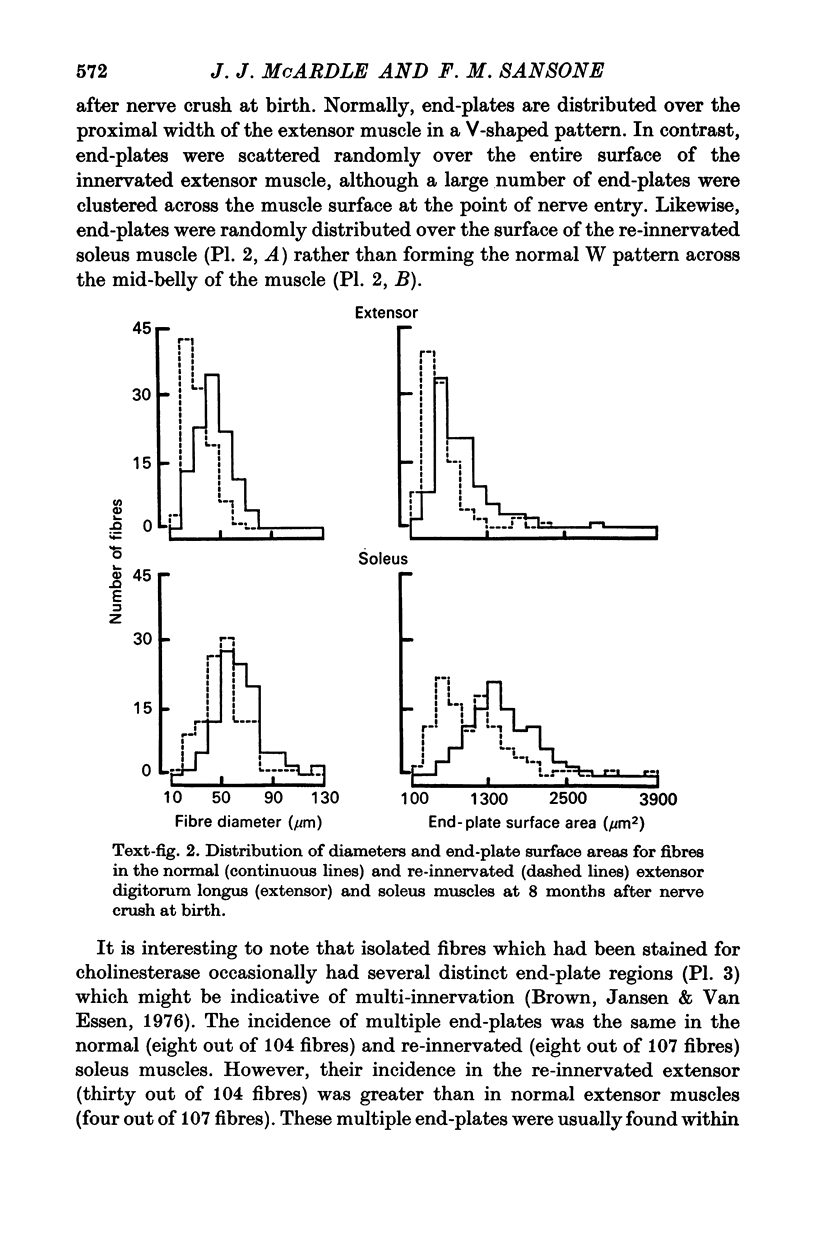
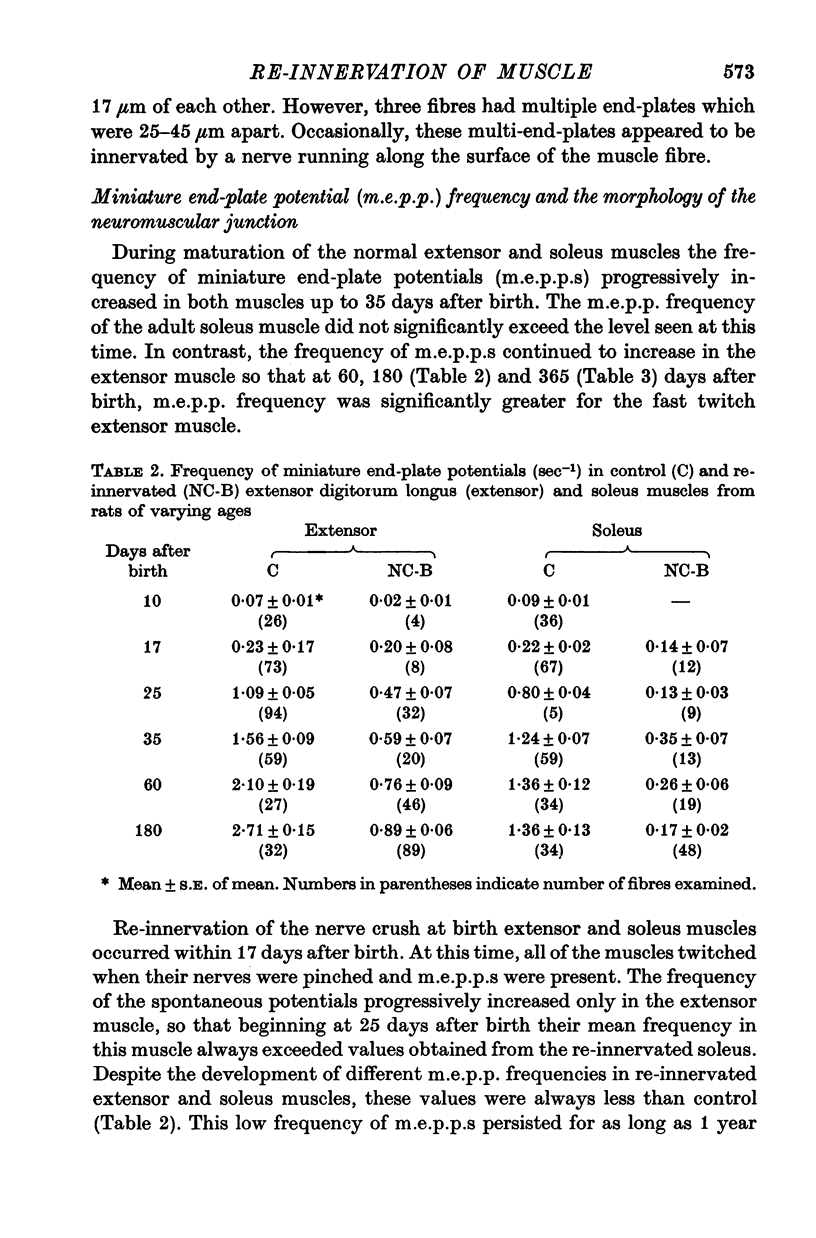
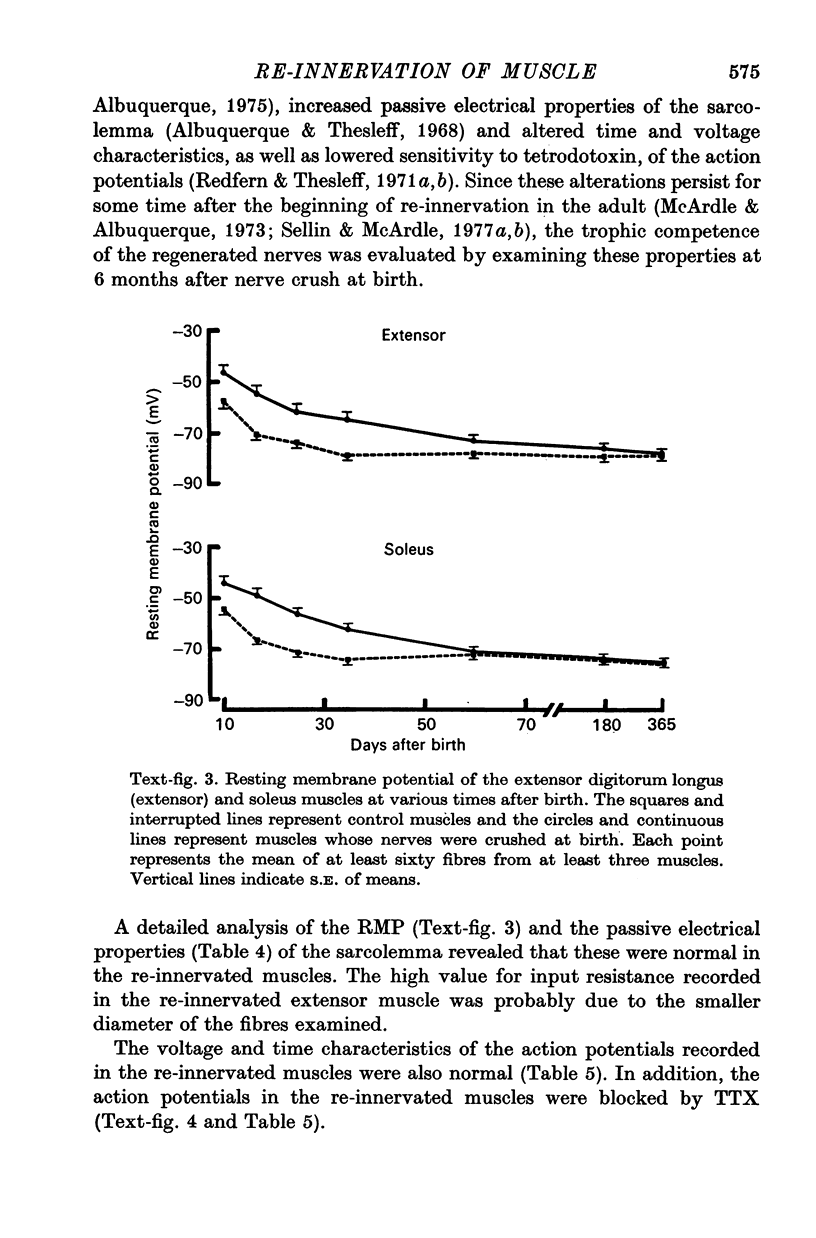
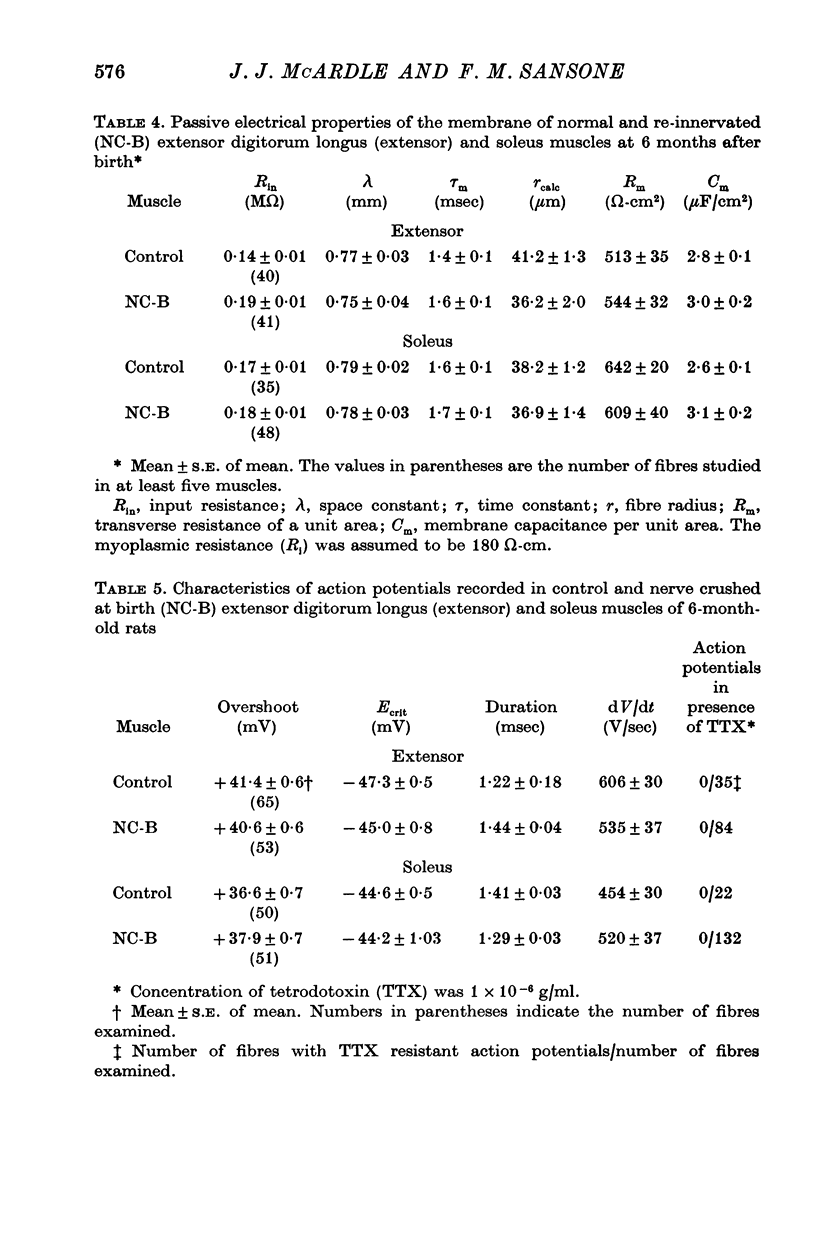
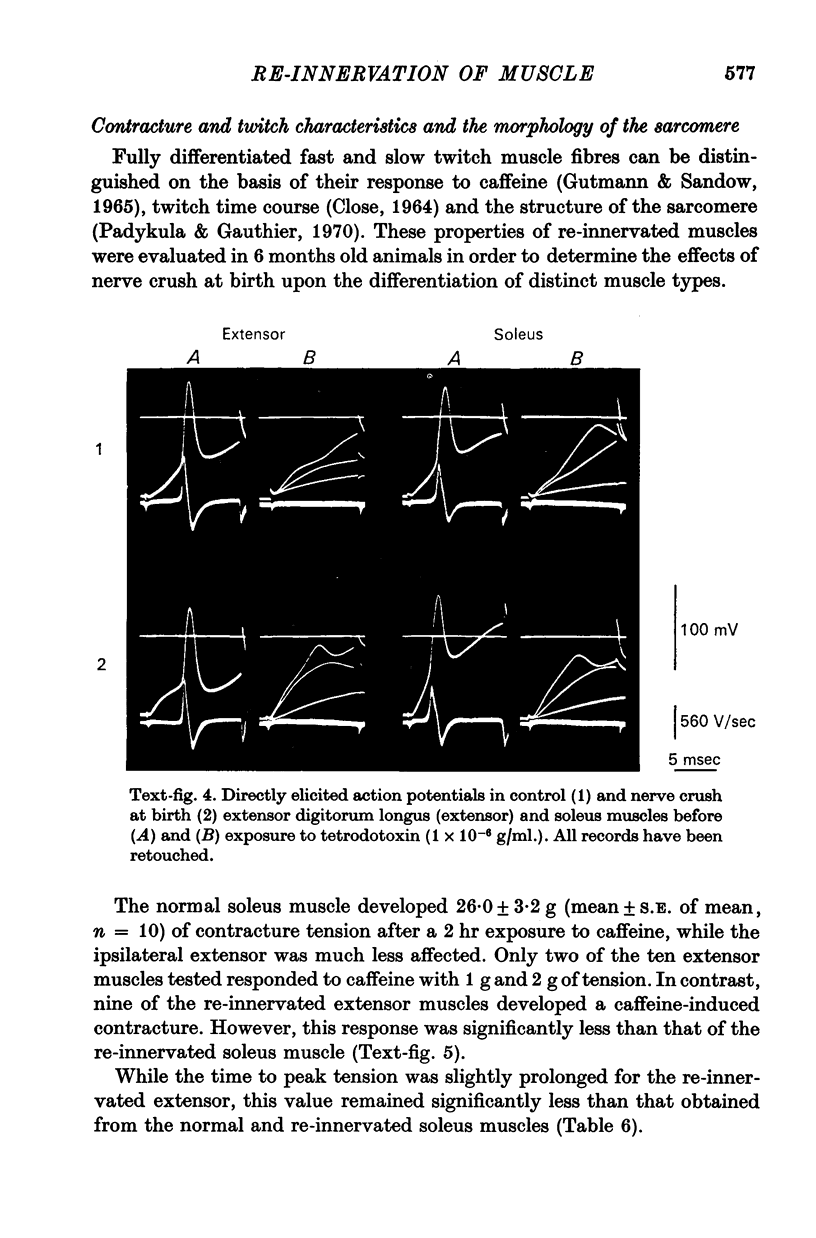
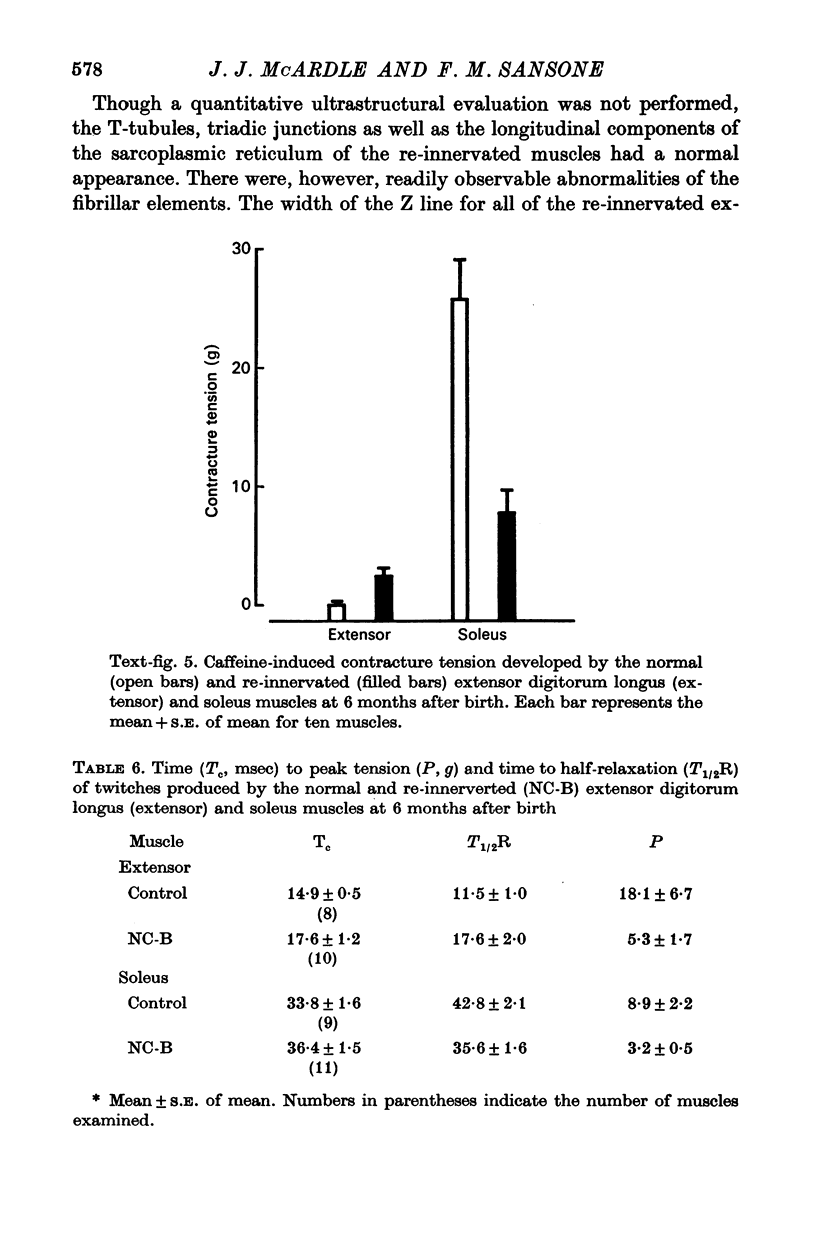
1. The frequency of miniature end-plate potentials (m.e.p.p.s) was significantly greater in the fast twitch extensor digitorum longus muscle (extensor) than in the slow twitch soleus, even though end-plate surface area was greater for fibres in the latter muscle. 2. Crush of the sciatic nerve at birth did not prevent the appearance of this difference in m.e.p.p. frequency. However, the frequency of the potentials in the re-innervated muscles was less than normal, even though the regenerated neuromuscular junction was qualitatively normal in morphology. 3. Though the re-innevated muscles were differentiated with respect to twitch time course, the extensor muscle was more responsive than normal to the contracture-inducing action of caffeine. 4. The Z line of the re-innervated extensor muscle was similar to that of the normal soleus in thickness. 5. Resting potential, passive electrical properties and action potential generating mechanism of the sarcolemma were normal. 6. Since the re-innervated muscles lacked muscle spindles, a role of sensory feed-back in the function of the neuromuscular junction as well as the neutrotrophic regulation of muscle is discussed.
Full text
PDF














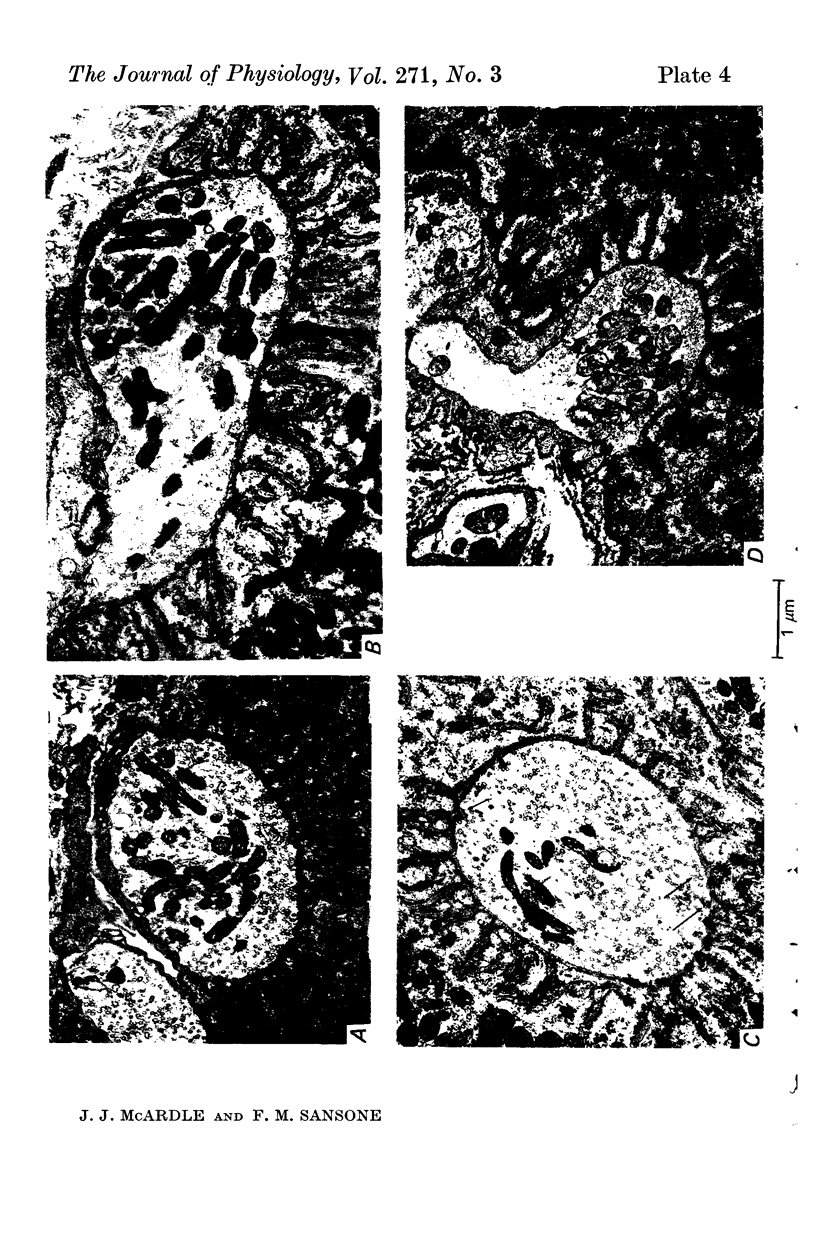

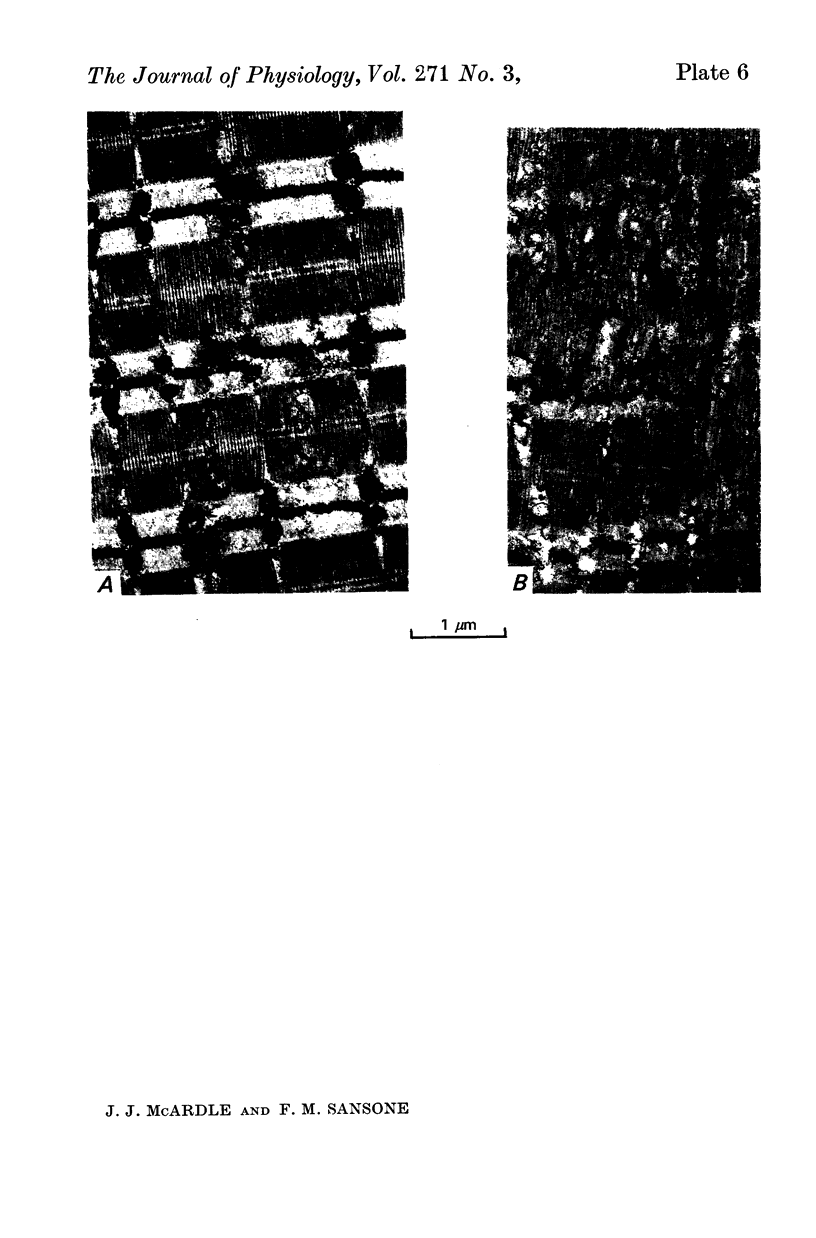
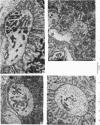
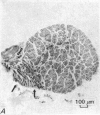
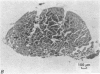
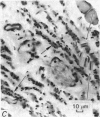
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W. A., Stromer M. H., Goll D. E., Suzuki A. Ca 2+ -specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972 Feb;52(2):367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Courrège P., Danchin A. A theory of the epigenesis of neuronal networks by selective stabilization of synapses. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2974–2978. doi: 10.1073/pnas.70.10.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Fulthorpe J. J. Stages in fibre breakdown in Duchenne muscular dystrophy. An electron-microscopic study. J Neurol Sci. 1975 Feb;24(2):179–200. doi: 10.1016/0022-510x(75)90232-4. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K., MILEDI R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959 Jan 28;145(1):204–220. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., McINTYRE A. K. The effects of disuse and of activity on mammalian spinal reflexes. J Physiol. 1953 Sep;121(3):492–516. doi: 10.1113/jphysiol.1953.sp004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W. K., Karpati G. Impaired skeletal muscle maturation following neonatal neurectomy. Dev Biol. 1968 Jun;17(6):713–723. doi: 10.1016/0012-1606(68)90015-8. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Sandow A. Caffeine-induced contracture and potentiation of contraction in normal and denervated rat muscle. Life Sci. 1965 Jun;4(11):1149–1156. doi: 10.1016/0024-3205(65)90104-9. [DOI] [PubMed] [Google Scholar]
- Hník P., Lessler M. J. Changes in muscle spindle activity of the chronically de-efferented gastrocnemius of the rat. Pflugers Arch. 1973 Jun 26;341(2):155–170. doi: 10.1007/BF00587322. [DOI] [PubMed] [Google Scholar]
- Hoh J. F. Selective and non-selective reinnervation of fast-twitch and slow-twitch rat skeletal muscle. J Physiol. 1975 Oct;251(3):791–801. doi: 10.1113/jphysiol.1975.sp011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizar P., Kuno M., Miyata Y. Differentiation of motoneurones and skeletal muscles in kittens. J Physiol. 1975 Nov;252(2):465–479. doi: 10.1113/jphysiol.1975.sp011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson A., Sandow A. Caffeine effects on radiocalcium movement in normal and denervated rat skeletal muscle. J Pharmacol Exp Ther. 1967 Feb;155(2):376–388. [PubMed] [Google Scholar]
- KOZAK W., WESTERMAN R. A. Plastic changes of spinal monosynaptic responses from tenotomized muscles in cats. Nature. 1961 Mar 4;189:753–755. doi: 10.1038/189753b0. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969 Jul;42(1):154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke S., Solomon H. C. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J Exp Zool. 1967 Dec;166(3):377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Mark R. F. Selective innervation of muscle. Br Med Bull. 1974 May;30(2):122–126. doi: 10.1093/oxfordjournals.bmb.a071181. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. Effects of ouabain on denervated and dystrophic muscles of the mouse. Exp Neurol. 1975 May;47(2):353–356. doi: 10.1016/0014-4886(75)90263-0. [DOI] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- McComas A. J., Mrozek K. Denervated muscle fibres in hereditary mouse dystrophy. J Neurol Neurosurg Psychiatry. 1967 Dec;30(6):526–530. doi: 10.1136/jnnp.30.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H. Y., Kuncl R. W., Yang V. Incidence of Z band streaming and myofibrillar disruptions in skeletal muscle from healthy young people. Neurology. 1976 Sep;26(9):853–857. doi: 10.1212/wnl.26.9.853. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E. Non-selective re-innervation of slow and fast muscle fibres in the rat. Nature. 1969 May 10;222(5193):569–571. doi: 10.1038/222569a0. [DOI] [PubMed] [Google Scholar]
- Padykula H. A., Gauthier G. F. The ultrastructure of the neuromuscular junctions of mammalian red, white, and intermediate skeletal muscle fibers. J Cell Biol. 1970 Jul;46(1):27–41. doi: 10.1083/jcb.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Ridge R. M. The differentiation of conduction velocities of slow twitch and fast twitch muscle motor innervations in kittens and cats. Q J Exp Physiol Cogn Med Sci. 1967 Jul;52(3):293–304. doi: 10.1113/expphysiol.1967.sp001915. [DOI] [PubMed] [Google Scholar]
- Robbins N., Fischbach G. D. Effect of chronic disuse of rat soleus neuromuscular junctions on presynaptic function. J Neurophysiol. 1971 Jul;34(4):570–578. doi: 10.1152/jn.1971.34.4.570. [DOI] [PubMed] [Google Scholar]
- Robbins N., Nelson P. G. Tenotomy and the spinal monosynaptic reflex. Exp Neurol. 1970 Apr;27(1):66–75. doi: 10.1016/0014-4886(70)90202-5. [DOI] [PubMed] [Google Scholar]
- Sellin L. C., McArdle J. J. Colchicine blocks neurotrophic regulation of the resting membrane potential in reinnervating skeletal muscle. Exp Neurol. 1977 May;55(2):483–492. doi: 10.1016/0014-4886(77)90016-4. [DOI] [PubMed] [Google Scholar]
- Sellin L. C., McArdle J. J. Effect of ouabain on reinnervating mammalian skeletal muscle. Eur J Pharmacol. 1977 Feb 7;41(3):337–340. doi: 10.1016/0014-2999(77)90328-4. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Asiedu S. A., Milhorat A. T. Effect of neonatal neurectomy on differentiation of fiber types in rat skeletal muscle. Exp Neurol. 1972 Jun;35(3):529–540. doi: 10.1016/0014-4886(72)90123-9. [DOI] [PubMed] [Google Scholar]
- Stauber W. T., Schottelius B. A. Calcium uptake by subcellular fractions of denervated anterior and posterior latissimus dorsi muscles. Exp Neurol. 1975 Sep;48(3 Pt 1):534–541. doi: 10.1016/0014-4886(75)90011-4. [DOI] [PubMed] [Google Scholar]
- Teräväinen H. Development of the myoneural junction in the rat. Z Zellforsch Mikrosk Anat. 1968;87(2):249–265. doi: 10.1007/BF00319723. [DOI] [PubMed] [Google Scholar]
- Teräväinen H., Juntunen J. Effect of temporary denervation on the develop- ment of the acetylcholinesterase-positive structures of the rat myoneural junction. Histochemie. 1968;15(3):261–269. doi: 10.1007/BF00305890. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Vyskocil F., Ward M. R. The action potential in end-plate and extrajunctional regions of rat skeletal muscle. Acta Physiol Scand. 1974 Jun;91(2):196–202. doi: 10.1111/j.1748-1716.1974.tb05676.x. [DOI] [PubMed] [Google Scholar]