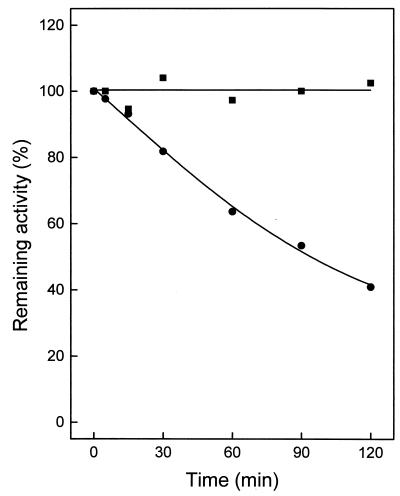
FIG. 3.
Thermostability of ATP-GLK from A. pernix. Enzyme (1 μg purified from A. pernix) was incubated in 40 μl of 50 mM potassium phosphate buffer (pH 7.0) at 95°C (▪) and at 100°C (•). At the times indicated, samples were cooled on ice for 10 min and assayed for the remaining activity at 90°C in the direction of G6P formation. One hundred percent activity corresponded to a specific activity of 35 U/mg.