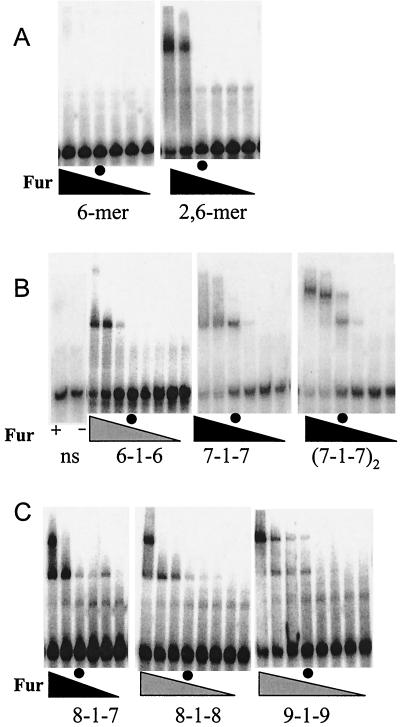
FIG. 2.
Binding of B. subtilis Fur to model oligonucleotide substrates. The final concentrations of Fur protein (monomer) in the reaction mixtures were 0, 1, 10, 100, 500, and 1,000 nM in the panels with six lanes (solid triangles) and 0, 10, 50, 75, 100, 200, 500, and 1,000 nM in the panels with eight lanes (shaded triangles). For ease of comparison between experiments, the lanes containing 100 nM Fur (monomer) are indicated by circles. (A) Binding of Fur protein to oligonucleotides containing either one or two copies of the GATAAT hexamer motif. (B) Comparison of Fur protein binding to the 6-1-6, 7-1-7, and (7-1-7)2 substrates. A nonspecific DNA fragment (ns) (see Table 2) was included as a control with either 1 μM Fur (lane +) or no added Fur (lane −). (C) Effect of additional flanking bases on formation of the lower-mobility complex (see Table 2 for a summary). Note that these studies were not done in parallel with those whose results are shown in panel B, so the absolute affinities cannot be compared.