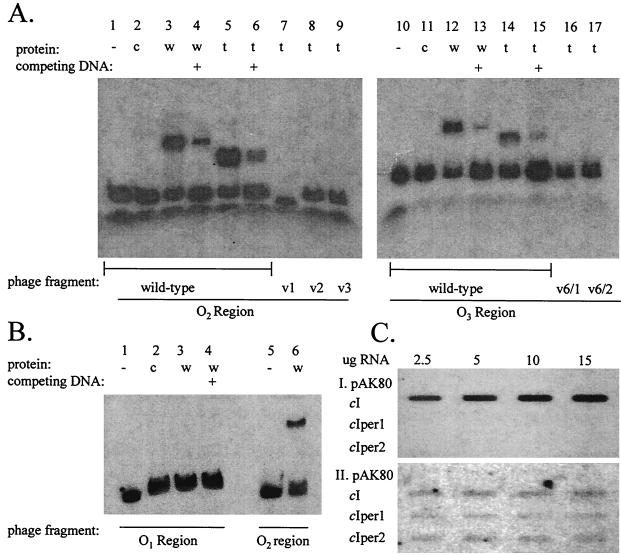
FIG. 5.
(A) Gel mobility shift assay showing binding to operators O2 and O3. Lanes 1 to 6, O2 fragment from wild-type phage φ31; lanes 7, 8, and 9, O2 fragments from the mutated φ31 variants (v) 1, 2, and 3, respectively; lanes 10 to 15, O3 fragment from wild-type phage φ31; lanes 16 and 17, O3 fragment from double-mutant φ31 variants (v) 6/1 and 6/2, respectively. Protein was produced in single-tube transcription-translation reactions using either the wild-type (w) φ31 cI gene (added to lanes 3, 4, 12, and 13) or the truncated (t) cI gene (added to lanes 5 to 9, and 14 to 17). Lanes 2 and 11 contain added control (c) transcription-translation reaction mixture lacking a cI template. Lanes 4, 6, 13, and 15 (indicated with a +) contain competing unlabeled O2 or O3 probe DNA. (B) Gel mobility shift assay showing wild-type CI binding. Lanes 1 to 4, O1 fragment from wild-type phage φ31; lanes 5 and 6, O2 fragment from the phage; wild-type CI protein was added in lanes 3, 4, and 6. Competing unlabeled O1 fragment DNA was added in lane 4 (indicated with a +). Lane 2 contains added control (c) transcription-translation reaction mixture lacking a cI template. (C) RNA slot blot probed with 32P-labeled PCR product from the P1 transcript (I) and from the start of the P2 transcript (II). Total RNA was isolated from NCK203 cells containing pAK80 and the indicated inserts in pAK80.