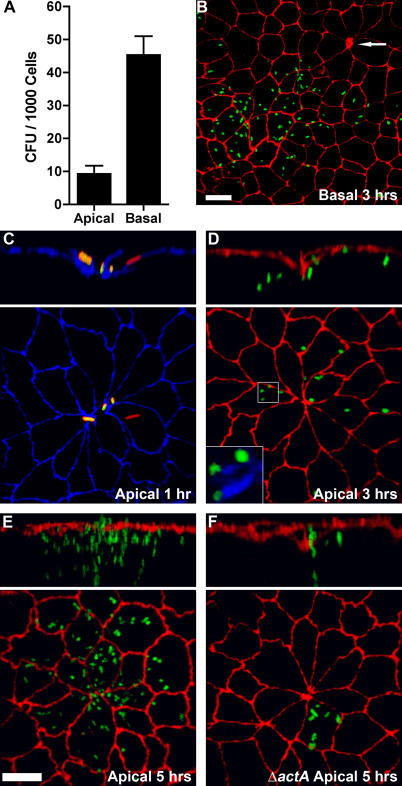
Figure 1. Invasion and Replication of L. monocytogenes at Multicellular Junction Sites.
Polarized MDCK monolayers on Transwell filters were infected from the apical (A, C–F) or basal (A and B) sides with L. monocytogenes.
(A) Viable CFUs of intracellular L. monocytogenes were determined after gentamicin treatment. Means and standard deviations from quadruplicate samples are shown. Sample groups are significantly different: unpaired t-test p < 0.0001.
(B–D) Three-dimensional reconstructions of confocal immunofluorescence images of the invasion sites. Upper panels are reconstructions of Z-sections.
(B) At 3 h after basal infection, foci of replication were visualized with antibodies to L. monocytogenes (green) and ZO-1 (red). Arrow indicates a multicellular junction site.
(C) At 1 h after apical infection, a representative site of invasion was visualized with antibodies to ZO-1 (blue). To evaluate intracellular versus extracellular bacteria, we performed an inside/outside staining where extracellular adherent L. monocytogenes were stained before permeabilization in green and both intracellular and extracellular bacteria were stained after permeabilization in red. External L. monocytogenes thus appear as a combination of red/green or yellow.
(D) At 3 h after apical infection, sites of replication were visualized with antibodies to L. monocytogenes (green) and ZO-1 (red), and with phalloidin (blue) to show actin comet-tails in association with the cytoplasmic bacteria, inset.
(E) At 5 h after apical infection, foci of replication and spread were visualized with antibodies to ZO-1 (red) and L. monocytogenes (green).
(F) Using the same methodology as in (E), monolayers were infected with ΔactA L. monocytogenes (green), which are capable of cell invasion and intracellular replication but not cell-to-cell spread.
Scale bars 10 μm.