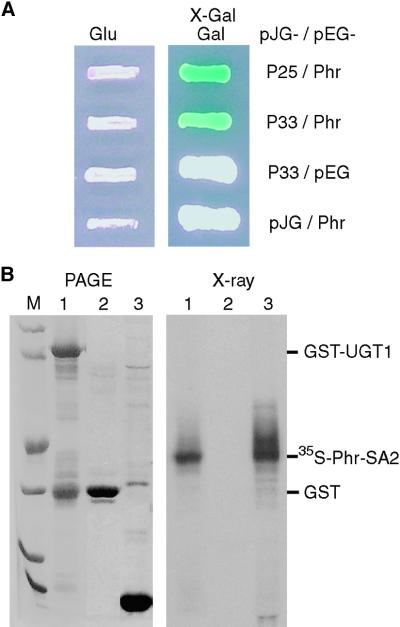
Figure 1.
Interaction between Phragmoplastin and UGT1.
(A) Interaction in the yeast two-hybrid system. Using soybean phragmoplastin (Phr; Gu and Verma, 1996) as a bait to screen an Arabidopsis cDNA library, two independent clones (pPIL25 [P25] and pPIL33 [P33]) were pulled out and later confirmed by sequencing to represent the same gene. Cloning vectors pEG202 (pEG) and pJG4-5 (pJG) were used as negative controls. Yeast colonies containing the plasmids were grown on SC-Ura-Trp-His+Leu medium in the presence of glucose (Glu) or galactose (Gal) and X-Gal.
(B) Pull-down assay of in vitro transcription/translation products. In lane 1, UGT1 was expressed as a glutathione S-transferase (GST)–tagged protein in Escherichia coli and purified using glutathione–agarose beads. The beads were incubated with 35S-labeled phragmoplastin, proteins bound to the beads were resolved by PAGE (left), and then the same gel was dried and exposed to x-ray film (right). Note that it is not unusual to see a GST band copurified with a GST-tagged recombinant protein. In lane 2, GST expressed from vector pGEX-KG served as a negative control and was treated as described for lane 1. In lane 3, plasmid pAGA3-SA2 expressing the SA2 domain of phragmoplastin (Phr-SA2; Zhang et al., 2000) was used to synthesize 35S-labeled phragmoplastin in an in vitro transcription/translation system. The positions of the proteins are marked. Lane M contains standard protein markers of (from bottom to top) 14, 21, 31, 45, 66, and 97 kD.