Figure 4.
Dependence of AKT3/(p)KST1 and KST1/(p)AKT3 on Extracellular Ca2+ and H+.
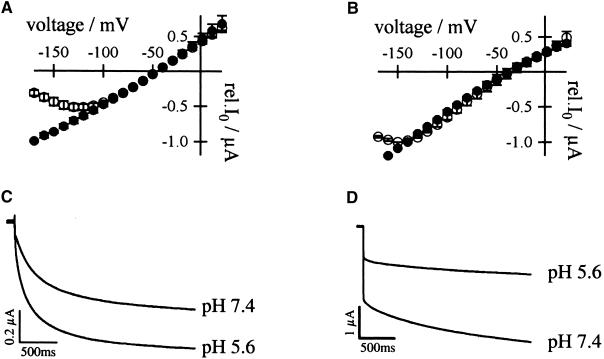
(A) Relative (rel.) instantaneous tail-current amplitudes I0 plotted against the membrane voltage revealed a voltage-dependent Ca2+ block for AKT3 (○, 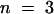 ) but not for the chimeric channels AKT3/(p)KST1 (•,
) but not for the chimeric channels AKT3/(p)KST1 (•, 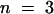 ). The Ca2+ solution contained 20 mM KCl, 10 mM Tris/Mes, pH 7.2, and 30 mM CaCl2. In the control solution, CaCl2 was replaced with 30 mM MgCl2. Error bars were smaller than symbols and represent the standard deviation (n ⩾ 3). I0 currents in the presence of calcium were significantly different in the voltage range of −130 to −170 mV for the chimera AKT3/(p)KST1 compared with AKT3 wild type (P < 0.01).
). The Ca2+ solution contained 20 mM KCl, 10 mM Tris/Mes, pH 7.2, and 30 mM CaCl2. In the control solution, CaCl2 was replaced with 30 mM MgCl2. Error bars were smaller than symbols and represent the standard deviation (n ⩾ 3). I0 currents in the presence of calcium were significantly different in the voltage range of −130 to −170 mV for the chimera AKT3/(p)KST1 compared with AKT3 wild type (P < 0.01).
(B) Superimposed I0/V-plot of KST1 and KST1/(p)AKT3 . Under the same experimental conditions as described in (A), the chimera KST1/(p)AKT3 was blocked by extracellular Ca2+ (○, 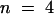 ) in contrast to KST1 wild type (•,
) in contrast to KST1 wild type (•, 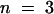 ). Error bars were smaller than symbols and represent the standard deviation (n ⩾ 3). I0 currents in the presence of calcium were significantly different in the voltage range of −150 to −170 mV for the chimera KST1/(p)AKT3 compared with KST1 wild type (P < 0.01).
). Error bars were smaller than symbols and represent the standard deviation (n ⩾ 3). I0 currents in the presence of calcium were significantly different in the voltage range of −150 to −170 mV for the chimera KST1/(p)AKT3 compared with KST1 wild type (P < 0.01).
(C) Acid-activated inward K+ currents of AKT3/(p)KST1 in response to 2.5-sec voltage pulses to −150 mV from the holding voltage of −20 mV. Currents were recorded in the presence of standard external media buffered to pH values as indicated.
(D) Voltage pulses to −150 mV from the holding voltage of −20 mV elucidated that K+ currents of KST1/(p)AKT3 decreased upon a change from pH 7.4 to 5.6.
(C) and (D) show representative current traces out of four independent experiments. Steady state currents in (C) and (D) at pH 5.6 were significantly different from currents at pH 7.4 at −150 mV (P < 0.01).