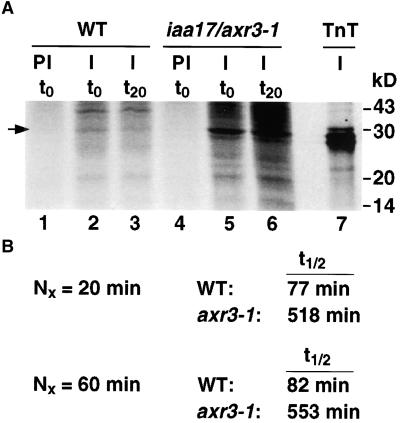
Figure 2.
Pulse–Chase Analysis of the IAA17/AXR3 Protein in Wild-Type and iaa17/axr3-1 Plants.
(A) Intact 7-day-old etiolated seedlings were labeled with 35S-methionine for 2 hr. The tissue was rinsed and either harvested (t0, lanes 1, 2, 4, and 5) or allowed to incubate in a buffer containing a 1000-fold excess of cold methionine and cysteine for 20 min (t20, lanes 3 and 6). The tissue was frozen in liquid N2, ground, and lyophilized. Proteins were extracted and immunoprecipitated with protein A–purified preimmune serum (PI, lanes 1 and 4) or with affinity-purified anti-IAA17/AXR3 antibody (I, lanes 2, 3, 5, 6, and 7). The protein sample in lane 7 was generated by a coupled in vitro transcription and translation (TnT) reaction in the presence of 35S-methionine using the IAA17/AXR3 cDNA as template. The precipitated proteins were separated by SDS-PAGE and detected using the STORM phosphorimager system. The arrow indicates the position of IAA17/AXR3. Numbers at the right denote molecular mass in kilodaltons (kD).
(B) Determination of the half-life (t1/2) of IAA17/AXR3 and iaa17/axr3-1. In two separate experiments, the amount of radioactivity present in the immunoprecipitated IAA17/AXR3 bands at t0 or after either a 20- min (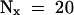 ) or 60-min (
) or 60-min (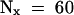 ) chase period was determined using the STORM phosphorimager system. These values were used to calculate the half-lives of IAA17/AXR3 and iaa17/axr3-1 based on the formula
) chase period was determined using the STORM phosphorimager system. These values were used to calculate the half-lives of IAA17/AXR3 and iaa17/axr3-1 based on the formula 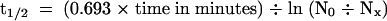 , where N0 is the amount of protein at t0 and Nx is the amount of protein remaining after a given chase time.
, where N0 is the amount of protein at t0 and Nx is the amount of protein remaining after a given chase time.