FIG. 2.
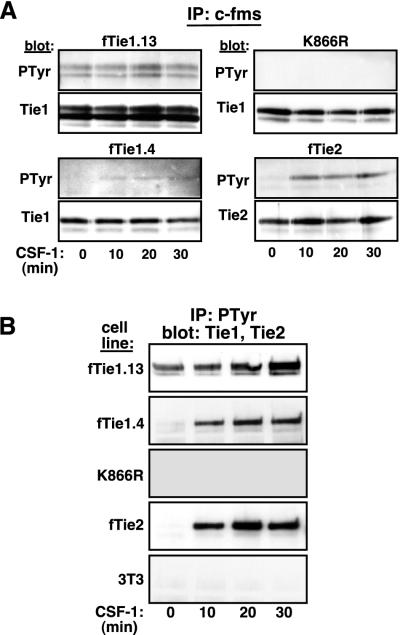
Tie1 is autophosphorylated in vivo. (A) Autophosphorylation of Tie1 is kinase dependent. Two independent clones of NIH 3T3 cells expressing wild-type fTie1 as well as cells expressing a kinase-inactive mutant of fTie1 (K866R) or fTie2 were serum starved and were then treated with CSF-1 (500 ng/ml) for 0, 10, 20, or 30 min. Lysates from these cells were immunoprecipitated with anti-c-fms, separated by SDS-8% PAGE, and Western blotted with antiphosphotyrosine (PTyr, clone PY99), and then stripped and reprobed with anti-Tie1 or anti-Tie2. Weak but detectable increases in ligand-induced tyrosine phosphorylation were observed in both fTie1-expressing clones but not in K866R cells. As a control, fTie2 cells showed readily detectable ligand-induced autophosphorylation. (B) Tie1 is present in antiphosphotyrosine immunoprecipitates. The cells used for panel A, as well as untransfected 3T3 cells, were treated as described above, except lysates were immunoprecipitated with antiphosphotyrosine (PY99) and were then Western blotted with anti-Tie1 or (for fTie2 cell lysates) anti-Tie2. Tie1 was readily detectable in antiphosphotyrosine immunoprecipitates from both low- and high-receptor-expressing fTie1 cells but not from K866R cells or untransfected 3T3 cells. CSF-1 treatment also resulted in the presence of Tie2 in phosphotyrosine immunoprecipitates.