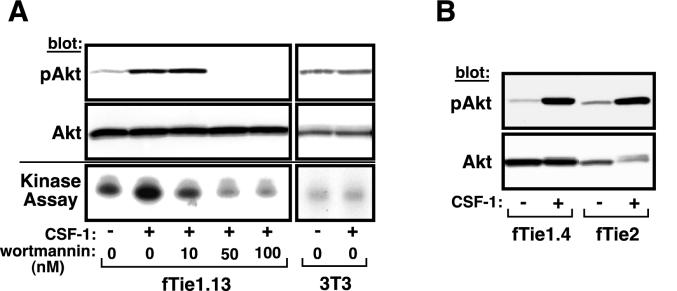
FIG. 4.
Tie1 induces phosphorylation and activation of Akt. (A) fTie1-expressing cells or untransfected NIH 3T3 cells were serum starved overnight and were then left untreated or were treated 8 min with CSF-1 (500 ng/ml) in the presence of 1 mM vanadate and the indicated concentration of wortmannin or an equal volume of DMSO. An aliquot of each cell lysate was separated by SDS-8% PAGE and Western blotted with Abs specific for phosphoserine 473 of Akt (pAkt) or total Akt (upper panels). The remainder of each sample was immunoprecipitated with anti-Akt and was used in an in vitro kinase reaction with [γ-32P]ATP and recombinant histone H2B as a substrate. Radiolabeled histone H2B was separated by SDS-15% PAGE and evaluated by autoradiography (lower panel). (B) A second independent clone of fTie1-expressing cells as well as fTie2 and K866R cells (not shown) was treated as described for panel A, and cell lysates were Western blotted sequentially with anti-phospho-Akt and anti-Akt.