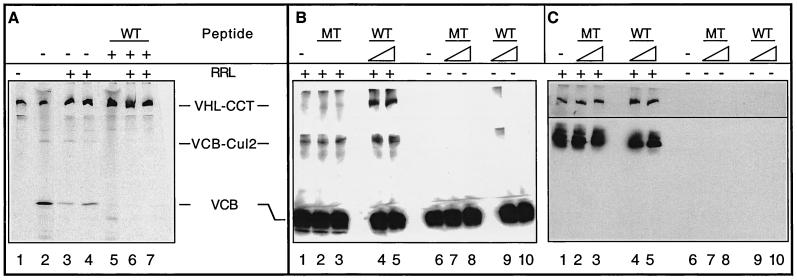
FIG. 3.
A role for CCT in the assembly and disassembly of VCB. (A) Generation of VCB from purified VHL-CCT complexes. VHL-CCT complexes assembled in RRL were isolated by gel filtration chromatography and analyzed by native-PAGE (lane 1). To mark the position of migration of the VCB and VCB-Cul2 complexes, a sample of the unfractionated RRL reaction also was analyzed (lane 2). Purified VHL-CCT was incubated in the presence of RRL and an ATP-regeneration system (lanes 3 and 4), which resulted in the appearance of both VCB and VCB-Cul2. The migration position of VHL-CCT is marked by pVHL synthesized in the presence of the elongin C binding peptide (lane 5). When purified VHL-CCT was incubated in RRL in the presence of the elongin C binding peptide, VCB and VCB-Cul2 formation now was blocked (lanes 6 and 7). (B and C) pVHL present in the VCB complex rebinds to CCT upon VCB disassembly. (B) Recombinant VCB (see Materials and Methods) was added to rabbit reticulocyte lysate (+RRL, lanes 1 to 5) and then incubated in the absence (lane 1) or presence of either the pVHL peptide (WT, lanes 4 and 5) or the mutant pVHL peptide (MT, lanes 2 and 3) described in Fig. 2C (either 120 or 600 μM as indicated by the triangles). As a control, the recombinant VCB was incubated in the absence (lane 6) or presence of either wild type (WT, lanes 9 and 10) or mutant (MT, lanes 7 and 8) peptide in the absence of RRL (−RRL, lanes 6 to 10). After 60 min of incubation, the reaction products were analyzed by native-PAGE. The proteins in the gel were denatured, transferred to nitrocellulose, and analyzed in panel B by Western blotting with a monoclonal antibody specific for pVHL. The positions of the pVHL-containing complexes are indicated at the left. (C) The membrane from the blot shown in panel B was stripped and reprobed with anti-CCT-1α (top) or anti-Cul2 antibodies (bottom). The lane designations are the same as for panel B.