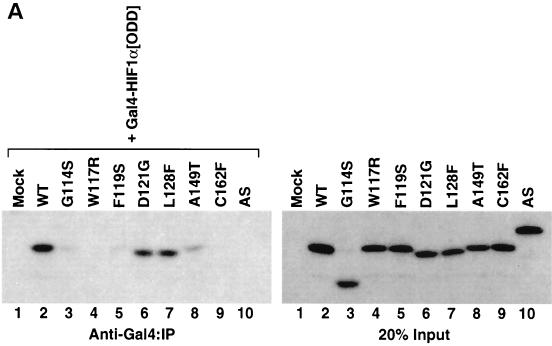
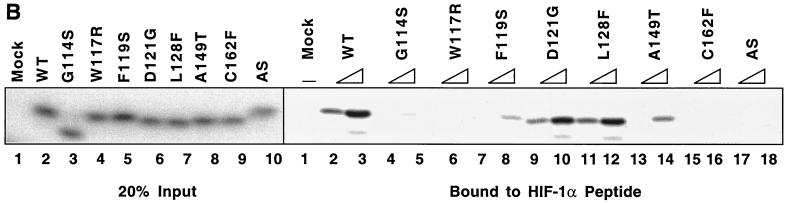
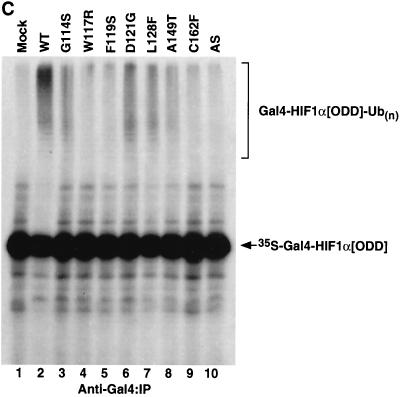
FIG. 6.
Diverse effects of mutations in exon II of VHL on ability of VCB-Cul2 to interact with the HIF-1α sequence implicated in oxygen-dependent proteolysis. (A) Wild-type and mutant VHL mRNAs were translated in the RRL in the presence of [35S]methionine. In a separate reaction, Gal4-HIF-1α[ODD] mRNA was translated (in the absence of [35S]methionine). Equal portions of the two reactions were mixed together in order to allow the 35S-labeled VCB-Cul2 to interact with the Gal4-HIF-1α[ODD] substrate. After a 30-min incubation, the Gal4-HIF-1α[ODD] was captured by using an anti-Gal4 antibody bound to protein A-Sepharose. The amount of 35S-labeled pVHL coprecipitating was determined by SDS-PAGE and fluorography. In the right panel, 20% of the input radiolabeled pVHL proteins used in the analysis are shown. In the left panel, the radiolabeled pVHL proteins immunoprecipitated by the anti-Gal4 antibodies are shown. The particular pVHL polypeptide synthesized in each reaction is indicated at the top of the panel. Note that the G114S mutant pVHL (lane 3) was observed to migrate faster than the wild-type (lane 2) and that the exon II deletion mutant (lane 10) migrated slightly slower than the wild-type protein under these conditions during SDS-PAGE in Tris-glycine buffer. The reason for this anomalous migration is not known. These anomalies were not observed when Tris-Tricine gels were used. (B) Binding of pVHL encoded by exon II mutants to a hydroxylated HIF-derived peptide. The indicated wild-type and mutant VHL mRNAs were translated in RRL in the presence of [35S]methionine. Then, 5 or 10 μl of radiolabeled translation products, as indicated by the triangles, was incubated with 0.1 μg of an immobilized biotin-conjugated hydroxylated HIF-derived peptide (a substrate for the VCB-Cul2 complex) (see Materials and Methods) to allow 35S-labeled VCB-Cul2 to interact with the hydroxylated peptide. After a 1-h incubation at 4°C, the amount of bound 35S-labeled pVHL was determined by SDS-PAGE and fluorography (right panel). In the left panel, 20% of the input radiolabeled pVHL proteins used in the analysis is shown. “Mock” indicates a reaction that was programmed with empty plasmid. (C) The ability of VHL exon II mutants to stimulate the ubiquitination of HIF-1α correlates well with the binding of the pVHL variants to HIF-1α. In an experiment similar to that shown in panels A and B, Gal4-HIF-1α[ODD] was synthesized in RRL, this time in the presence of [35S]methionine. In parallel, the wild type and the different pVHL mutants were translated separately in the absence of radiolabel. Portions of the translation reactions (4 μl of 35S-labeled Gal4-HIF-1α[ODD] and 2 μl of each unlabeled pVHL) were mixed together, along with an in vitro system capable of ubiquitin conjugation (see Materials and Methods). The Gal4-HIF-1α[ODD] was isolated by immunoprecipitation with anti-Gal4 antibodies, and the resultant immunoprecipitates were analyzed by SDS-PAGE and fluorography. The particular pVHL polypeptide synthesized in each reaction is indicated at the top of the panel. Indicated near the top of the gel is the position of ubiquitin-conjugated Gal4-HIF-1α[ODD] [UB(n)]. “Mock” indicates a reaction that was programmed with empty plasmid.