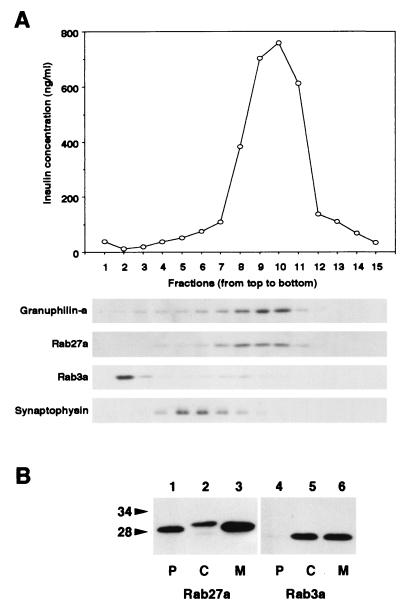
FIG. 4.
Subcellular localization of Rab27a and Rab3a. (A) A postnuclear supernatant of MIN6 cells was separated on a linear sucrose density gradient. Fifteen fractions were collected, and immunoreactive insulin in a portion of each fraction was measured (topmost panel). Equal volumes of the fractions were analyzed by immunoblotting using antibodies against granuphilin-a (79-kDa protein), Rab27a (28-kDa protein), Rab3a (26-kDa protein), and synaptophysin (36- to 38-kDa protein). (B) MIN6 cells were fractionated as described previously (5). Briefly, cells were harvested, swollen in hypotonic buffer (20 mM 3-morpholinopropanesulfonic acid [pH 7.3], 1 mM MgCl2, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride), and ruptured by passage through a 26-gauge needle. After centrifugation at 100,000 × g for 60 min, the supernatant (C; soluble cytosolic fraction) was precipitated with 10% trichloroacetic acid plus 0.1% sodium deoxycholate. The pellet was resuspended in hypotonic buffer plus 1% Nonidet P-40. The suspension was centrifuged at 10,000 × g for 10 min to separate the supernatant (M; detergent-soluble membrane fraction) and the pellet (P; detergent-insoluble particulate fraction). Equal proportions of the fractions were separated on a polyacrylamide gel for immunoblotting. Numbers to the left of panel B are molecular masses in kilodaltons.