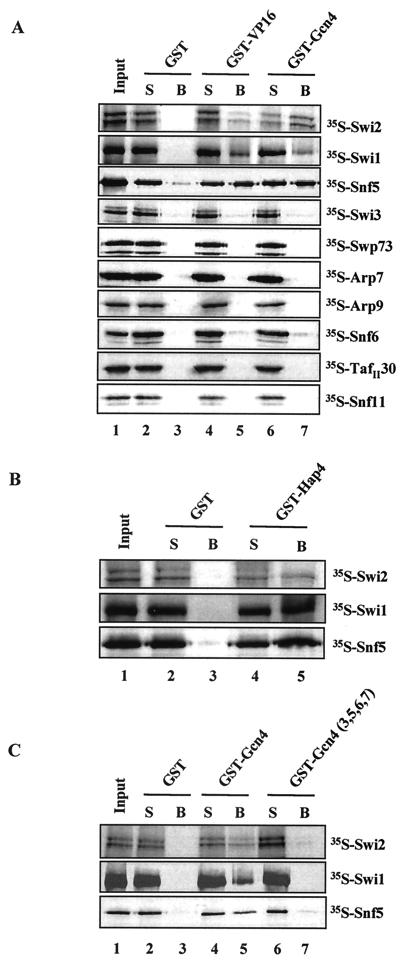
FIG. 5.
Interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators but not with activation domain mutants by GST pull-down analysis. (A) GST pull-down assays were performed with the GST fusion proteins indicated bound to glutathione-Sepharose beads and individually expressed 35S-labeled SWI/SNF subunits. A total of 50% of each input and supernatant (S) and 100% of the proteins associated with the beads (B) were loaded on SDS-8, 10, or 12% polyacrylamide gels, depending on the size of the labeled subunit. The gels were fixed, enhanced, and dried, and radiolabeled proteins were visualized by autoradiography. (B) GST pull-down assays were performed as for panel A, with GST or GST-Hap4 (amino acids 330 through 554) and the 35S-labeled SWI/SNF subunits indicated. (C) GST pull-down as-says were performed as for panels A and B, with the GST fusion proteins and 35S-labeled SWI/SNF subunits indicated. Gcn4[3,5,6,7] contains mutations in hydrophobic residues within its activation domain that reduce its activation potential.