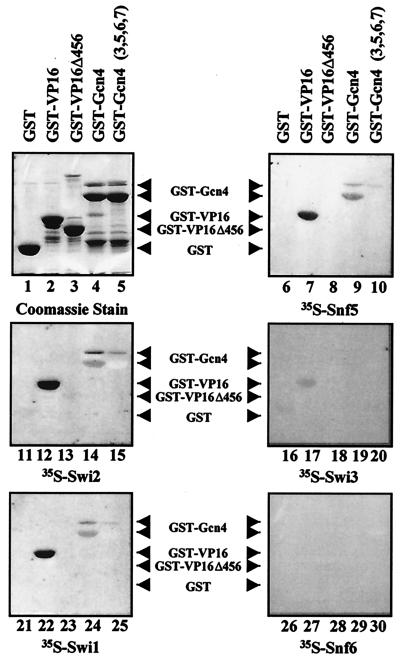
FIG. 6.
Interaction of Snf5, Swi1, and Swi2/Snf2 with acidic activators but not with activation domain mutants by far-Western analysis. Far-Western analysis was used to confirm the interactions of Snf5, Swi1, and Swi2/Snf2 with GST-VP16 and GST-Gcn4. GST fusion proteins were run on a SDS-10% polyacrylamide gel and transferred to PVDF membrane. After denaturation and renaturation steps, the blots were incubated with in vitro transcription and translation rabbit reticulocyte lysate reactions containing the 35S-labeled SWI/SNF subunit indicated below each panel. A COOH-terminally truncated VP16 activation domain (GST-VP16Δ456) and full-length Gcn4 that contained hydrophobic cluster mutations in its activation domain (GST-Gcn4 [3,5,6,7]) were also tested for interaction with the 35S-labeled subunits. The interaction of the radiolabeled subunits with the GST fusions on the blot was visualized by autoradiography.