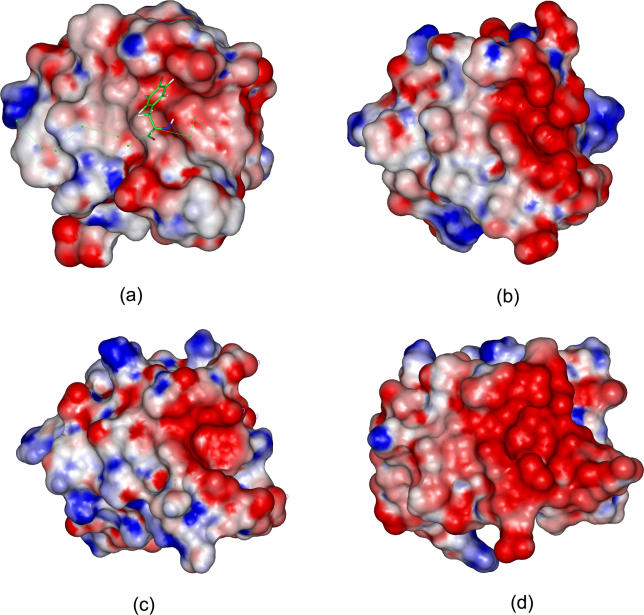
Figure 4. The Electrostatic Potentials of the Peptide-Binding Interfaces for Four SH3 Domains.
(A) 1bbz, (B) 1cka, (C) 1gbq, and (D) 1bb9.
The scale of gradation was from −5 kT/e to +5 kT/e corresponding to red color to blue color. The electrostatic potentials of proteins were calculated using the Delphi module in Insight II. The salt concentration was set to 0.0 M because electrostatic potentials had small changes in the range of the experimental salt concentrations. The internal and external dielectric constants were set to 1 and 80, respectively. Electrostatic potentials were computed using a grid space of 0.5 Å with the focusing technique. The structures of the four SH3 domains were aligned using the Homology module in Insight II. The Tyr residue at P−3 in peptide APSYSPPPPP was shown in stick.