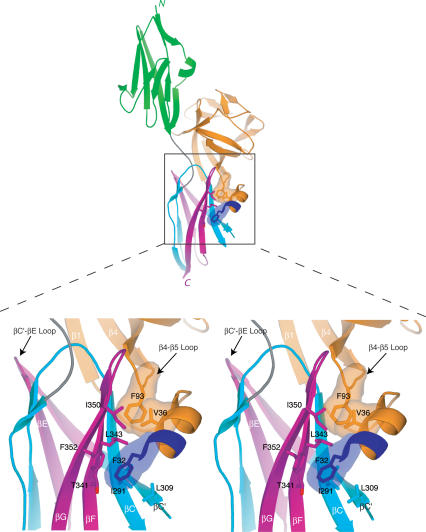
Figure 3.
Contacts between the N-terminal helix and β4-β5 loop of FGF8b with the D3 hydrophobic groove of FGFR2c dictate FGF8-FGFR-binding specificity. Interactions are shown in stereo and coloring is the same as in Figure 1A. The molecular surfaces of the FGF8b residues that interact with the D3 groove are shown. Note that F32 is the only residue from the alternatively spliced region of FGF8b that interacts with FGFR. Note that the βC′-βE loop of FGFR2c (marked by an arrowhead) does not interact with FGF8b.