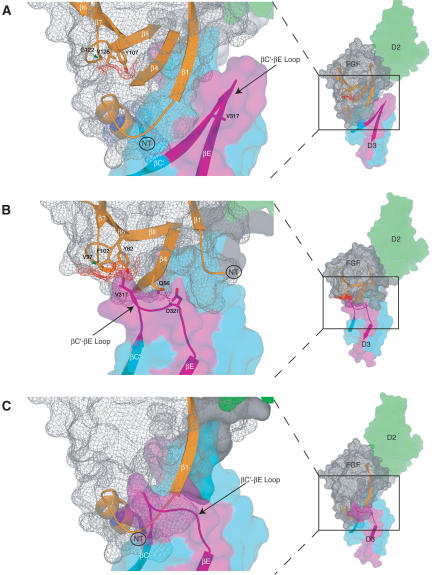
Figure 7.
The structural mechanism by which FGF8b attains its receptor-binding specificity deviates from the more general mode described for FGF1, FGF2, and FGF10. Comparison of the overall mode of the FGF-D3 interaction observed in the FGF8b-FGFR2c (A) and in the FGF2-FGFR2c (PDB identification code 1EV2) (B) structures. The FGF2-FGFR2c structure is representative of the overall mode of receptor binding previously determined for FGF1, FGF2, and FGF10. In each panel, FGFR is colored as in Figure 1A, and the molecular surface of FGF is shown as a gray mesh. In B, unique hydrogen bonds between the βC′-βE loop of receptor and FGF2 that play a key role in determining FGF2-FGFR2c specificity are represented by dashed lines. The hydrophobic patch of FGF2 that interacts with the βC′-βE loop of receptor (B) and the corresponding patch of FGF8b (A) are shown in red mesh. The reduction of the red mesh area in FGF8b reflects the fact that G122 of FGF8b replaces V97 of FGF2 (indicated by green arrows). (C) The FGF8b mode of binding is incompatible with that of other FGFs. FGF8b from the FGF8b-FGFR2c structure was superimposed onto FGF2 in the FGF2-FGFR2c structure. Note that the N terminus of FGF8b runs directly into the βC′-βE loop of D3 due to opposite spatial positioning of the FGF8b N terminus relative to the β-trefoil core, compared with that of other FGFs (cf. the locations of the N termini of FGF8b and FGF2 in A and B; also refer to Fig. 1C).