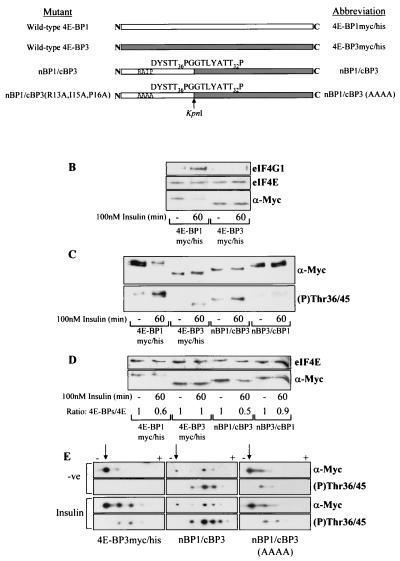
FIG. 7.
Insulin-induced phosphorylation of 4E-BP3 is increased when 4E-BP3 contains the N terminus of 4E-BP1. (A) Diagrammatic representation of mutants used. White and grey boxes, 4E-BP1 and 4E-BP3 proteins, respectively. These mutants each possess C-terminal Myc and His tags (not shown; see Fig. 2A). Arrow, KpnI site used in creating the chimeras. The amino acid sequence expressed adjacent to this site within each chimera is indicated. The phosphorylation sites in 4E-BP1 (Thr36 and -45) and the corresponding sites in 4E-BP3 (Thr23 and -32) are indicated. (B) Cell samples from HEK293 cells overexpressing tagged 4E-BP1 or 4E-BP3 were subjected to affinity chromatography on m7GTP-Sepharose as described for Fig. 2C. (C) HEK293 cells expressing either 4E-BP1myc/his, 4E-BP3myc/his, nBP1/cBP3, or nBP3/cBP1 were stimulated with insulin as indicated. The extracts were analyzed using anti-Myc antiserum (α-Myc) to detect the levels of expression and using 4E-BP1 phospho-Thr36/45 antiserum to analyze phosphorylation of 4E-BP3 at Thr23 and 4E-BP1 at Thr36/45. (D) The cell extracts from panel C were subjected to affinity chromatography with m7GTP-Sepharose. The ratios of recombinant 4E-BP protein bound to eIF4E (4E-BPs/4E) were determined by densitometric analysis of the blots, where the ratios during serum-starved conditions are normalized to 1. (E) HEK293 cells overexpressing either 4E-BP3myc/his, nBP1/cBP3, or nBP1/cBP3(AAAA) were treated with insulin as indicated. The cell extracts were analyzed by isoelectric focusing as described in Materials and Methods. The overexpressed protein was analyzed using anti-Myc antiserum and the 4E-BP1 phospho-Thr36/45 antiserum (to analyze phosphorylation of 4E-BP3 at Thr23 and of 4E-BP1 at Thr36/45). Arrows, positions of the most basic, least phosphorylated form of each protein that lies closest to the cathode (−). Upon phosphorylation the 4E-BP3 protein migrates further toward the anode (+), and so the observed shift toward the anode from the point of origin is representative of increasing phosphorylation.