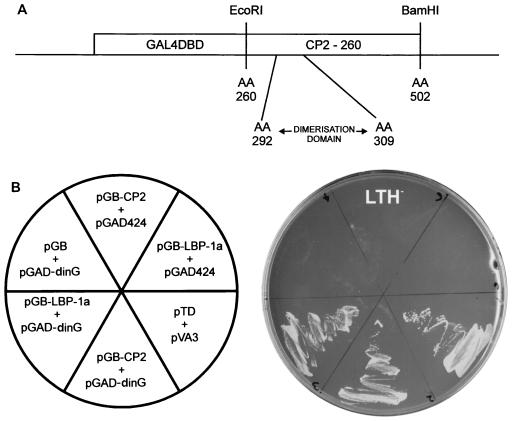
FIG. 1.
Isolation of the CP2-interacting protein, dinG. (A) Schematic of the bait construct used in the yeast two-hybrid screen of a K562 cDNA library. The coding sequence of CP2 from amino acids (AA) 260 to 502 were fused in frame with the GAL4 DNA binding domain (GAL4DBD). The minimal dimerization domain (amino acids 292 to 309) is shown. (B) Human dinG interacts specifically with CP2 and LBP-1a in yeast. (Left) The Saccharomyces cerevisiae reporter strain HF7C was transformed with the indicated plasmids. pGB-LBP-1a contains the carboxy-terminal half of LBP-1a analogous to pGB-CP2-260. pGAD-dinG was the original clone identified in the library screen and contains a dinG fragment encoding amino acids 257 to 336. pGB, pGAD424, and the positive controls pTD and pVA3 have been reported previously. (Right) Transformants were streaked onto synthetic medium plates lacking tryptophan, leucine, and histidine (LTH−) and incubated at 30°C for 3 days.