Abstract
Objective:
To compare the clinical, microbiological, and therapeutic features of nonpostoperative nosocomial intra-abdominal infections (non-PostopNAI) with community-acquired intra-abdominal infections (CAI).
Summary Background Data:
Prospective (June 2000 through January 2001) consecutive case series analysis of patients operated for secondary nonpostoperative intra-abdominal infections collected in 176 study centers (surgical wards and intensive care units).
Patients and Methods:
Clinical, microbiological, and therapeutic characteristics of CAI and non-PostopNAI infections were collected. Management of antibiotic therapy was decided by the attending physician. The efficacy of treatment was evaluated over a 30-day period after the index episode.
Results:
Evaluatable observations (n = 1008) were collected (761 CAI and 247 non-PostopNAI), including 285 intensive care unit patients. When compared with CAI patients, non-PostopNAI patients presented an increased interval between admission to the surgical ward and operation (1.3 ± 1.5 vs. 0.5 ± 0.7 days in CAI patients; P < 0.001), increased proportions of underlying diseases, a more severe clinical condition as assessed by increased proportions of hospitalization in the intensive care unit (48% vs. 22% in CAI patients, P < 0.001) and a higher SAPS II score (34 ± 15 vs. 24 ± 14, P < 0.001). In non-PostopNAI patients, increased proportions of therapeutic failure (15% vs. 7% in CAI patients, P < 0.01) and of fatalities (12% vs. 4% in CAI patients, P < 0.001) were observed.
Conclusions:
Delayed diagnosis and increased severity are the main characteristics of non-PostopNAI infections. Microbiological features are quite similar in CAI and non-PostopNAI infections, suggesting that antibiotic therapy recommended for CAI infections could be applied to non-PostopNAI patients. Characteristics of non-PostopNAI patients should lead to identify them as a specific entity in clinical trials.
Clinical, microbiological, and therapeutic characteristics of community-acquired and nonpostoperative nosocomial intra-abdominal infections were compared in a prospective case series analysis collecting 1008 observations of secondary infections in 176 study centers. Delayed diagnosis, increased severity, and poorer outcome were observed in patients who underwent an operation for nonpostoperative nosocomial intra-abdominal infections.
Over the last 2 decades, many publications has addressed the issue of the management of intra-abdominal infections in many fields, such as surgical techniques, intensive care unit (ICU) management, and antimicrobial therapy. However, certain subgroups of patients have been totally forgotten from clinical investigations, such as those with nonpostoperative nosocomial intra-abdominal infections. The term nosocomial is somewhat confusing because many physicians believe that nosocomially acquired infection is that which results after surgery. The general definition of nosocomial intra-abdominal infection puts together 2 different diseases, postoperative infection and nonpostoperative infection.1 In nosocomial nonpostoperative infection, the infectious process is not present on admission and becomes evident 48 hours or more after admission, without any surgical intervention.1 There are several clinical circumstances in which this diagnosis can be made in patients hospitalized for a reason other than intra-abdominal infection. These include elderly patients, patients receiving immunosuppressive drugs such as steroids, patients with cardiovascular or respiratory failure, diabetic patients, and, occasionally patients from medical care or long-term care facilities.
A review of the literature shows that clinical trials either omitted to mention the origin of the patients or considered non postoperative intra-abdominal infections to be the same as postoperative cases.2–4 Physicians have to assume that these infections share several clinical and microbiological characteristics in common with other nosocomial infections due to preoperative hospitalization and colonization with nosocomial flora.5 However, these patients might also present a number of similarities with community-acquired intra-abdominal infections, such as absence of previous surgery or similar etiologies of intra-abdominal infection.
A better description of clinical practice could help to improve the management of these patients, by defining therapeutic strategies assessed according to the type of patient and the severity of the disease. A large multicenter epidemiological study was therefore conducted to assess the clinical, microbiological and therapeutic features in patients undergoing surgery for secondary intra-abdominal infections, excluding postoperative patients. Special attention was focused on the differences between patients operated for community-acquired intra-abdominal infections (CAI) and nonpostoperative nosocomial intra-abdominal infections (non-PostopNAI).
PATIENTS AND METHODS
Study Population
This study was performed between June 2000 and January 2001 throughout France. To obtain a representative sample of French hospital institutions, 187 physicians in 176 study centers working in 40 teaching hospitals and 136 nonteaching hospitals participated in the study. Institutional review board approval was obtained for the study. In each study center, a physician identified as the center coordinator, collected the data.
All patients included in this prospective study were adults (≥15 years) of either sex operated for nonpostoperative intra-abdominal infection. All patients received intravenous antibiotic therapy prescribed by the attending physician for this diagnosis.
Subjects with any of the following disease states were excluded from the study: postoperative peritonitis, female genital tract infection or perinephric infection; simple acute nonperforated appendicitis; traumatic perforation of small or large bowel operated within 12 hours of the perforation; perforated gastric or duodenal ulcer less than 24 hours old; transmural necrosis of the intestine caused by acute embolic or thrombotic occlusion; simple acute nonperforated cholecystitis with infection confined to the gallbladder; spontaneous bacterial peritonitis; peritonitis associated with chronic peritoneal dialysis; need for “open abdomen” management or surgical “zippers.” In addition, patients not receiving antibiotic therapy were excluded from the study. Transplant recipients and cases diagnosed at autopsy were also excluded. Antimicrobial therapy administered before the diagnosis of intra-abdominal infection for another indication was not considered to be an exclusion criterion.
The methods used for diagnosis were determined by the attending physicians and corresponded to the procedures applied in their institutions. Similarly, the microbiological procedures corresponded to the procedures routinely used in the laboratory. For each microbiological sample collected, the investigators reported the results of culture and bacterial identification and susceptibility testing. Surgical management was decided by the attending surgeon. Antibiotics, changes of therapy and duration of treatment were decided by the attending physician. The reasons for changes of antimicrobial therapy were reported.
Data Collection
The following items were prospectively recorded: demographic data, underlying disease,6 length of preoperative hospital stay (in the case of nosocomial infection), SAPS II score at the time of inclusion,7 microbiological parameters (peritoneal samples and blood cultures), surgical management (origin of infection, procedures applied), antibiotic management (initial therapy, changes in therapy and their causes, duration of treatment), and outcome.
Definitions
Nosocomial intra-abdominal infection was defined as an infection not present on admission that becomes evident 48 hours or more after admission in patients hospitalized for a reason other than intra-abdominal infection.1 Patients were defined as being potentially immunosuppressed when corticosteroids or radiotherapy or chemotherapy had been administered during the previous 6 months, or in the case of acquired immunodeficiency syndrome.
The parameters collected were used to determine the number and types of organ dysfunction: cardiovascular dysfunction assessed by heart rate <30 or ≥140 bpm or systolic blood pressure <90 mm Hg; hematologic dysfunction assessed by a white blood cell count <2500 or >49,900/mm3; renal dysfunction assessed by blood urea nitrogen >20 mmol/L or urinary output <500 mL/day; neurologic dysfunction assessed by Glasgow coma score <6; respiratory dysfunction assessed by mechanical ventilation; and hepatic dysfunction assessed by serum bilirubin >34.2 μmol/L. Patients with a SAPS II score ≥38 at the time of diagnosis were arbitrarily considered to have severe forms of intra-abdominal infection.
Bacteremia was defined as at least one positive blood culture (2 positive blood cultures in the case of coagulase-negative staphylococci) isolated during the 2 days after the diagnosis. The type of antibiotic regimen used was noted such as triple-drug therapy, double combination or monotherapy. First-line beta-lactam antibiotics were defined as the following treatments: aminopenicillins ± beta-lactamase inhibitor, first- and second-generation cephalosporins, cephamycins, cefotaxime, ceftriaxone. Extended spectrum beta-lactams were defined as the following treatments: ticarcillin ± clavulanic acid, piperacillin ± tazobactam, imipenem/cilastatin, ceftazidime, cefepime, cefpirome.
The reasons for changes of antimicrobial therapy were defined according to the following categories: clinical failure or persistent infection, other associated infections, cultured organisms resistant to the initial antibiotic therapy, simplification of therapy, miscellaneous. Treatment was considered to be adequate when the antibiotics covered the bacteria cultured according to susceptibility results and inadequate when empirical treatment disregarded at least one pathogen. Interpretation of the choice of antibiotic as appropriate or inappropriate is strictly an interpretation with respect to the culture results obtained.
Outcome
The efficacy of treatment was evaluated over a 30-day period after the index episode. Success or failure of therapy for each episode was determined by standard criteria. Patients were deemed clinically cured if the patient was completely asymptomatic with respect to the original infection. All other situations corresponded to failure. Failures were reported by the investigator according to 4 categories: digestive complications, extradigestive complications, reoperation, or death. Cause of death was assessed by the investigator as related or not related to initial infection.
Statistical Analysis
Results are expressed as mean ± SD or proportions. Clinical and laboratory data were analyzed statistically with the χ2 test or Fisher exact test for comparison of proportions and analysis of variance for comparison of intergroup differences. Statistical significance was defined as P < 0.05.
RESULTS
Study Population
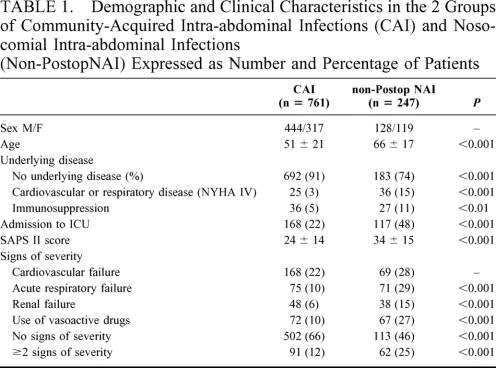
From the total of 1057 cases initially included, 49 were subsequently excluded (age <15 years in 11 cases, noncompliance with inclusion criteria or incoherent information in 38 cases), resulting in a total of 1008 evaluatable cases. Among the remaining 1008 evaluatable cases, 761 patients (75%) were classified as CAI, and 247 (25%) as non-PostopNAI (Table 1). One hundred twenty-eight (52%) of the non-PostopNAI patients were transferred from another ward and 56 (23%) from another hospital after a mean interval of 4 ± 5 days after admission, whereas 35 (14%) patients were referred from institutions (including 20 patients from medical care facilities). The mean interval between admission to the surgical ward and operation was 0.5 ± 0.7 days in CAI patients and 1.3 ± 1.5 days in non-PostopNAI patients (P < 0.001).
TABLE 1. Demographic and Clinical Characteristics in the 2 Groups of Community-Acquired Intra-abdominal Infections (CAI) and Nosocomial Intra-abdominal Infections (Non-PostopNAI) Expressed as Number and Percentage of Patients
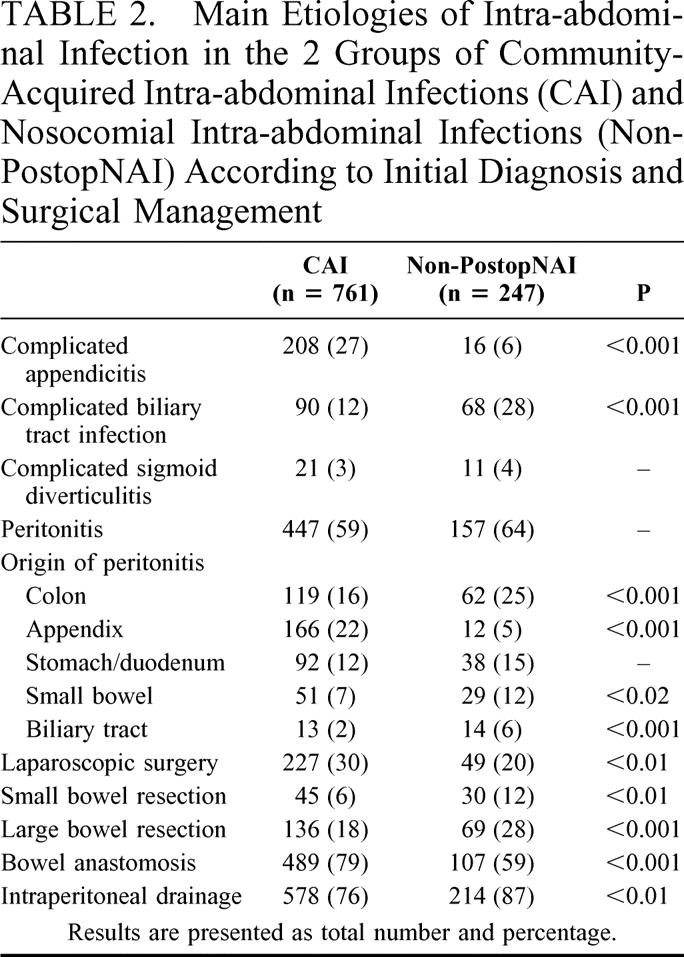
Clinical signs and signs of severity observed at the time of diagnosis are presented in Table 1. Analysis of the most severe patients revealed an increased proportion of non-PostopNAI patients (100 [41%] non-PostopNAI patients versus 106 [14%] CAI patients (P < 0.001). The etiologies of intra-abdominal infections (IAI) as assessed by surgical operation and surgical procedures are presented in Table 2.
TABLE 2. Main Etiologies of Intra-abdominal Infection in the 2 Groups of Community-Acquired Intra-abdominal Infections (CAI) and Nosocomial Intra-abdominal Infections (Non-PostopNAI) According to Initial Diagnosis and Surgical Management
Microbiological Results
Blood cultures were drawn in 540 patients but were positive in only 88 cases (30 [20%] non-PostopNAI patients). Two or more positive blood cultures were observed in 37 patients (12 non-PostopNAI patients). A total of 94 organisms were cultured (34 organisms in non-PostopNAI patients). The organisms most frequently cultured were staphylococci (n = 16, including 6 organisms in non-PostopNAI patients), Escherichia coli (n = 51, 13 organisms in non-PostopNAI patients), and Bacteroides spp (n = 14, 4 organisms in non-PostopNAI patients). Among the patients with positive blood cultures, no difference was observed in the type of pathogens cultured between CAI and non-PostopNAI patients.
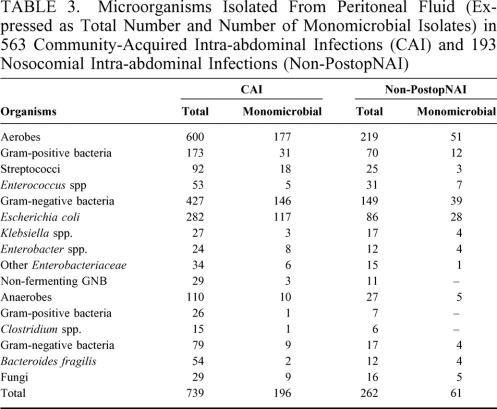
Surgical samples were obtained from 776 patients (573 CAI patients [76%] and 203 non-PostopNAI patients [82%], P < 0.05). A total of 1001 organisms were cultured from these samples in 756 of 776 patients (97%; 563 patients with CAI and 193 patients with non-PostopNAI; Table 3). In the 193 non-PostopNAI patients in whom microbiological cultures were performed, significantly higher proportions of enterococci were observed in polymicrobial cultures (31 enterococci among the 262 [12%] organisms isolated from non-PostopNAI infections vs. 53 of 739 [7%] organisms in CAI infections, P < 0.05). Similarly, the proportions of Gram-negative anaerobes observed in polymicrobial cultures were significantly higher in non-PostopNAI patients than in CAI patients (P < 0.05). The most frequent bacterial combinations were combinations of Gram-positive aerobic cocci and Gram-negative aerobic bacilli (249 of 367 [68%] CAI patients with polymicrobial infection vs. 87 of 132 [66%] non-PostopNAI patients), followed by combinations of Gram-negative aerobic bacilli and anaerobes (59 [16%] sampled CAI patients vs. 10 [7%] non-PostopNAI patients, P < 0.01) and Gram-positive aerobic cocci and anaerobes alone or associated to Gram-negative aerobic bacilli (59 [16%] sampled CAI patients vs. 6 [4%] non-PostopNAI patients, P < 0.001).
TABLE 3. Microorganisms Isolated From Peritoneal Fluid (Expressed as Total Number and Number of Monomicrobial Isolates) in 563 Community-Acquired Intra-abdominal Infections (CAI) and 193 Nosocomial Intra-abdominal Infections (Non-PostopNAI)
Among the 756 patients with positive samples, 143 (19%) of them demonstrated at least one organism with decreased susceptibility to the regimen administered (40 [5%] non-PostopNAI patients). One resistant organism was reported in 126 patients (34 non-PostopNAI patients), 2 or more resistant organisms in 12 patients (3 non-PostopNAI patients), and all organisms cultured were resistant to the treatment in 5 patients. Among these 151 resistant organisms, the most frequently reported strains were E. coli (n = 53, 13 cases in non-PostopNAI patients), other Enterobacteriaceae (n = 20, including 13 Enterobacter spp [4 in non-PostopNAI patients]), Pseudomonas spp (n = 13, 3 in non-PostopNAI patients), and enterococci (n=26, including 15 Enterococcus faecium [5 in non-PostopNAI patients]).
Among the 206 patients with severe infection, the proportion of patients with organisms resistant to the treatment administered was higher in non-PostopNAI patients (20 [10%] non-PostopNAI patients vs. 25 [4%] in CAI patients, P < 0.01).
Antibiotic Therapy
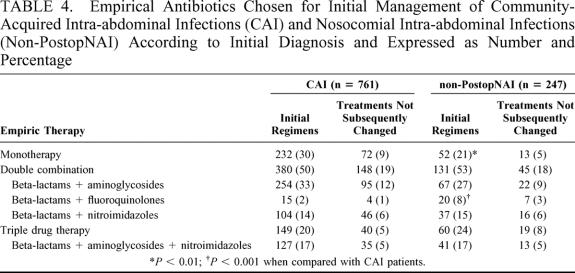
The regimens prescribed are summarized in Table 4. First-line beta-lactams were administered in 598 (79%) CAI patients and 151 (61%) non-PostopNAI patients (P < 0.001). Among monotherapies, first-line beta-lactams were more frequently administered in CAI patients (204 [27%] CAI patients vs. 36 [15%] non-PostopNAI patients, P < 0.001), mostly using aminopenicillins + beta-lactamase inhibitor (175 [23%] CAI patients vs. 29 [12%] non-PostopNAI patients, P < 0.001).
TABLE 4. Empirical Antibiotics Chosen for Initial Management of Community-Acquired Intra-abdominal Infections (CAI) and Nosocomial Intra-abdominal Infections (Non-PostopNAI) According to Initial Diagnosis and Expressed as Number and Percentage
Combination therapy was more frequently administered to the most severe patients (160 combinations [24%] vs. 45 monotherapies [14%], P < 0.001). However, no difference was observed in these proportions between CAI and non-PostopNAI patients. The proportion of extended spectrum beta-lactams was significantly higher in severe patients (126 [16%] low severity patients vs. 75 [41%] severe patients, P < 0.001) and the proportion of these drugs was higher in non-PostopNAI patients (administration of extended spectrum beta-lactams in 40 [62%] severe non-PostopNAI patients vs. 35 [34%] severe CAI patients, P < 0.001).
The mean time to change of treatment was 4 ± 4 days. The main reasons given for these changes were clinical failure or persistent infection (n = 88, including 36 [15%] non-PostopNAI patients, P < 0.001 when compared with CAI patients), organisms resistant to initial therapy (n = 101, 31 [13%] non-PostopNAI patients), simplification of therapy (n = 183, 41 [17%] non-PostopNAI patients), and infection of other origin (n = 28, 13 [5%] non-PostopNAI patients). In the group of 143 patients harboring resistant organisms, changes in antibiotic therapy were made in 81 patients (20 non-PostopNAI [50%] patients). Persistence of infection or clinical failure was observed in only 11 cases, including 1 non-PostopNAI patient. The duration of antibiotic therapy was longer in the group of patients treated for nosocomial infection (12 ±18 days in non-PostopNAI group vs. 9 ± 15 days in CAI patients, P < 0.01).
Patient Outcome
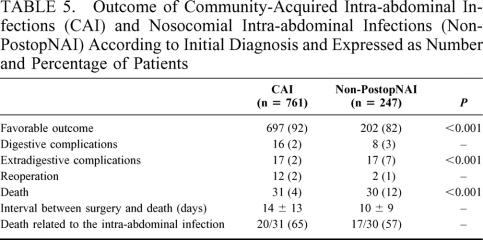
Patient outcome is presented in Table 5. Two hundred of the 286 patients admitted to ICU spent more than 2 days in ICU (109 [65%] CAI patients and 91 [78%] non-PostopNAI patients, P < 0.02) for a mean duration of 11 ± 10 days (range, 3–56). Forty-six (22%) of the most severe patients died (23 non-PostopNAI patients [23%]), and the proportion of digestive and extradigestive complications was similar in these patients (18% of postoperative complications).
TABLE 5. Outcome of Community-Acquired Intra-abdominal Infections (CAI) and Nosocomial Intra-abdominal Infections (Non-PostopNAI) According to Initial Diagnosis and Expressed as Number and Percentage of Patients
In the group of 143 patients with resistant organisms identified on susceptibility testing, a successful outcome was reported in 117 patients (82%; 32 non-PostopNAI [80%] patients), including the 5 patients in whom initial therapy did not target any of the cultured organisms. However, a trend toward increased morbidity was observed in these patients: digestive complications in 9 patients (6%), extradigestive complications in 9 patients (6%), and reoperation in 5 patients (3%). Fifteen (10%) of these 143 patients with resistant organisms died, including 5 non-PostopNAI patients.
DISCUSSION
To the best of our knowledge, our results represent the first large-scale epidemiological and prospective analysis of the management of secondary nonpostoperative intra-abdominal infections. This study also clarifies the clinical and therapeutic characteristics of nosocomial nonpostoperative intra-abdominal infections. Our results obviously cannot be compared with those of controlled randomized studies in view of several limitations related to the epidemiological nature of the study. However, we can assume that the data reported here present a comprehensive view of the difficulties encountered by clinicians in the management of these high-risk patients.
Although postoperative infections have been clearly assessed over the last 3 decades, nonpostoperative intra-abdominal infections have never been described or even defined. For instance, the Surgical Infection Society Guidelines on antimicrobial therapy for intra-abdominal infections published in 1992 did not mention this population.8 In the revised guidelines published in 2002, the nosocomial origin of intra-abdominal infection was considered in the antimicrobial therapy for the higher-risk patient but still without any definition.9 We assume that the best definition corresponds to the Centers for Disease Control criteria and may be applied in this setting like any other bacterial nosocomial infections.1
A wide range of therapeutic approaches is reported in this study, especially in terms of antibiotic therapy. There are several explanations for this diversity. Variability of medical practice can be at least partially explained by local technical conditions and limited availability of microbiology laboratories in several institutions. In a recent study conducted in French hospitals, clinicians reported access to a microbiology laboratory in only 50% to 90% of institutions with a laboratory open 24 hours a day, 7 days a week in only 70% of institutions.10 Second, the variability of microbiological isolates and regional or local variations in susceptibility could justify different therapeutic approaches. This issue has been previously addressed in the case of hospital-to-hospital variability of susceptibility of anaerobes. For instance, in 6 Chicago-area hospitals, clindamycin susceptibility of Bacteroides fragilis varied from 61% to 100%.11 Similar observations of regional variability have been reported with Enterobacteriaceae, mostly with E. coli and K. pneumoniae.12 Finally, the absence of reliable guidelines could also explain the wide range of therapeutic regimens reported here. Although many expert opinions have been published over the last 2 decades, no specific guidelines or consensus conferences were available at the time of initiation of this study. A French consensus conference was held during the period of the study, but neither the debates nor the published recommendations appeared to influence the investigators’ medical policy.13
Our data demonstrate that non-PostopNAI patients share a large number of demographic and clinical similarities with CAI patients. However, major clinical differences between these 2 groups include the high proportion of underlying disease and the marked severity at the time of diagnosis. The severity of the non-PostopNAI cases could be related to the underlying diseases per se but the delayed diagnosis reported in these patients might also play an important role. Several studies have demonstrated a striking correlation between delayed surgical treatment, number of medical and surgical complications and mortality.14–16 The site of infection and, consequently, the difficulties for diagnosis in non-PostopNAI patients, might also contribute to delayed surgery.15
Microbiological examinations were not performed in 23% of our patients, which raises the question of antibiotic susceptibility testing of bacterial isolates obtained from peritoneal infections.17 In a retrospective survey of 480 patients with secondary bacterial peritonitis, Mosdell et al18 reported only 68% of peritoneal sampling and noted that surgeons typically ignored culture data, as 9% of patients in this study had an appropriate change in antibiotic treatment after operation. Similarly, in complicated appendicitis, Dougherty et al19 noted that culture reports influenced antimicrobial therapy for only 7% of patients. In our study, technical problems raised by the limited availability of the microbiology laboratory in several institutions, and consequently the poor reliability of negative results, could have led clinicians to avoid microbiological sampling and to maintain their initial prescriptions. No conclusion can be drawn concerning the usefulness of microbiological sampling based on our data. However, it is noteworthy that several experts, including the French consensus conference on community-acquired peritonitis, have emphasized the need for routine susceptibility testing.13,20,21
A very limited number of studies have described the microbiological features of non-PostopNAI patients. In a study pooling nosocomial nonpostoperative and postoperative patients, Carlet et al2 reported a large proportion of Enterobacteriaceae and nonfermenting Gram negative anaerobes in non-PostopNAI patients, and one third of isolates demonstrated decreased susceptibility to antibiotic therapy. In a study focusing on the role of Candida in nosocomial and postoperative intra-abdominal infections, Calandra et al22 observed a high proportion of these organisms in non-PostopNAI patients. Unlike these studies, our results demonstrate large similarities between the microbiological characteristics of CAI and non-PostopNAI patients, and a low proportion of nosocomial flora. In addition, the rate of inadequate initial antibiotic therapy due to organisms with decreased susceptibility was low, suggesting that therapeutic recommendations for community-acquired intra-abdominal infections might also be appropriate in non-PostopNAI patients.
Monotherapies are widely used in the United States, as revealed by a study conducted in New Mexico, where the authors reported almost two thirds of monotherapies as initial regimens for acute bacterial peritonitis,18 whereas we reported only 28% of monotherapies. In our study, two thirds of empirical combination therapies involved aminoglycosides, while these authors reported administration of aminoglycosides in only 30% of cases.18 The use of aminoglycosides remains a source of debate.23,24 This issue was recently addressed in a French study demonstrating equivalent results with piperacillin/tazobactam alone or in combination with aminoglycosides.3 However, in the case of prescription of first-line beta-lactams, an approach adopted in 75% of cases in our study, administration of aminoglycosides is justified to cover the organisms involved. In a French study evaluating 300 amoxicillin-resistant E. coli isolates, high rates of resistance were observed to various first-line beta-lactams commonly used as empirical therapy.25 However, other Gram-negative organisms frequently isolated from community-acquired infections might also demonstrate decreased susceptibility toward these agents and justify combination therapy.20,26
The mortality rate reported in our study is situated in the low range, even in the most severe patients.27 The large number of patients presented here could more closely reflect the real prognosis of these patients than previous studies performed on limited numbers of cases. The similar mortality rates in the most severe non-PostopNAI and CAI patients must be stressed, suggesting that the type of infection in these secondary nonpostoperative infections might play only a minor role in the prognosis while the role of underlying diseases and of delayed diagnosis might be pivotal.14,15 This assertion is confirmed by similar proportions of death related to intra-abdominal infections in the 2 groups of patients, supporting the fact that intra-abdominal infection is only contributory to death. Aggressive treatments and supportive care together with admission in ICU might also attenuate the harmful effects of these important determinants of death.16 It is also worth mentioning that even the most severe non-PostopNAI patients had a better prognosis than patients operated for postoperative peritonitis.28,29 This point clearly demonstrates that non-PostopNAI patients cannot be assimilated to this population of patients and leads to reconsider inclusion criteria of clinical trials.3
In summary, non-PostopNAI are frequently characterized as severe infections diagnosed lately in fragile patients. Because of the high frequency of organ failure, aggressive treatments and supportive management in ICU are often required. Microbiological features are quite similar in CAI and non-PostopNAI infections, especially considering susceptibility of the cultured organisms toward the antimicrobial agents commonly administered. Our data suggest that antibiotic therapy recommended for CAI infections could be applied to non-PostopNAI patients. Finally, clinical and microbiological characteristics of these non-PostopNAI patients should lead to identify them as a specific entity in clinical trials.
Footnotes
Supported by a grant from GlaxoSmithKline France.
Reprints: Philippe Montravers, Département d'Anesthésie-Réanimation, CHU Jean Verdier, Avenue du 14 Juillet 93143 Bondy Cedex, France. E-mail: montravers@free.fr.
REFERENCES
- 1.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections. In: Olmsted RN, ed. APIC Infection Control and Applied Epidemiology: Principles and Practice. St Louis: Mosby; 1996:A-1–A-20. [Google Scholar]
- 2.Carlet J, Goldstein FW, Bleriot JP, et al. Timentin in the antimicrobial treatment of nosocomial and polymicrobial infections. J Antimicrob Chemother. 1986;17:149–159. [DOI] [PubMed] [Google Scholar]
- 3.Dupont H, Carbon C, Carlet J, et al. Monotherapy with a broad-spectrum beta-lactam is as effective as its combination with an aminoglycoside in treatment of severe generalized peritonitis: a multicenter randomized controlled trial. Antimicrob Agents Chemother. 2000;44:2028–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoeffel U, Jacobs E, Ruf G, et al. Intraperitoneal micro-organisms and the severity of peritonitis. Eur J Surg. 1995;161:501–508. [PubMed] [Google Scholar]
- 5.Sawyer RG, Rosenlof LK, Adams RB, et al. Peritonitis into the 1990s: changing pathogens and changing strategies in the critically ill. Am Surg. 1992;58:82–87. [PubMed] [Google Scholar]
- 6.McCabe WR, Jackson GG. Gram-negative bacteremia. I. Etiology and ecology. Arch Intern Med. 1962;110:847–864. [Google Scholar]
- 7.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPSII) based on a European-North American multicenter study. JAMA. 1993;270:29057–29068. [DOI] [PubMed] [Google Scholar]
- 8.Bohnen JM, Solomkin JS, Dellinger EP, et al. Guidelines for clinical care: anti-infective agents for intra-abdominal infection. Arch Surg. 1992;127:83–89. [DOI] [PubMed] [Google Scholar]
- 9.Mazuski JE, Sawyer RG, Nathens AB, et al. The Surgical Infection Society guidelines on antimicrobial therapy for intra-abdominal infections: evidence for the recommendations. Surg Infect. 2002;3:175–233. [DOI] [PubMed] [Google Scholar]
- 10.Montravers P, Veber B, Auboyer C, et al. Diagnostic and therapeutic management of nosocomial pneumonia in surgical patients: results of the Eole Study. Crit Care Med. 2002;30:368–375. [DOI] [PubMed] [Google Scholar]
- 11.Hecht DW, Osmolski JR, O'Keefe JP. Variation in the susceptibility of Bacteroides fragilis group isolates from six Chicago hospitals. Clin Infect Dis. 1993;16(suppl 4):S357–S360. [DOI] [PubMed] [Google Scholar]
- 12.Fluit AC, Jones ME, Schmitz FJ, et al. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30:454–460. [DOI] [PubMed] [Google Scholar]
- 13.Jury's recommendations. Management of community-acquired peritonitis—Consensus conference—Short text. Ann Fr Anesth Reanim. 2001;20:368s-373s. [PubMed] [Google Scholar]
- 14.Pitcher WD, Musher DM. Critical importance of early diagnosis and treatment of intra-abdominal infection. Arch Surg. 1982;117:328–333. [DOI] [PubMed] [Google Scholar]
- 15.Bohnen J, Boulanger M, Meakins J, et al. Prognosis in generalized peritonitis: relation to cause and risk factors. Arch Surg. 1983;118:285–290. [DOI] [PubMed] [Google Scholar]
- 16.Pacelli F, Doglietto GB, Alfieri S, et al. Prognosis in intra-abdominal infections. Multivariate analysis on 604 patients. Arch Surg. 1996;131:641–645. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblatt JE, Brook I. Clinical relevance of susceptibility testing of anaerobic bacteria. Clin Infect Dis. 1993;16:S446–S448. [DOI] [PubMed] [Google Scholar]
- 18.Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg. 1991;214:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dougherty SH, Saltzein EC, Peacock JB, et al. Perforated or gangrenous appendicitis treated with aminoglycosides. Arch Surg. 1989;124:1280–1283. [DOI] [PubMed] [Google Scholar]
- 20.Christou NV, Turgeon P, Wassef R, et al. Management of intra-abdominal infections. The case for intraoperative cultures and comprehensive broad-spectrum antibiotic coverage. Arch Surg. 1996;131:1193–1201. [DOI] [PubMed] [Google Scholar]
- 21.Wilson SE, Huh J. In defense of routine antimicrobial susceptibility testing of operative site flora in patients with peritonitis. Clin Infect Dis. 1997;25:S254–S257. [DOI] [PubMed] [Google Scholar]
- 22.Calandra T, Bille J, Schneider R, et al. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet. 1989;ii:1437–1440. [DOI] [PubMed] [Google Scholar]
- 23.Ho JL, Barza M. Role of aminoglycoside antibiotics in the treatment of intra-abdominal infection. Antimicrob Agents Chemother. 1987;31:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmen HP, Battaglia H, Kossmann T, et al. Effect of peritoneal fluid pH on outcome of aminoglycoside treatment of intraabdominal infections. World J Surg. 1993;17:393–397. [DOI] [PubMed] [Google Scholar]
- 25.Vanjak D, Muller-Serieys C, Picard B, et al. Activity of beta-lactamase inhibitor combinations on Escherichia coli isolates exhibiting various patterns of resistance to beta-lactam agents. Eur J Clin Microbiol Infect Dis. 1995;14:972–978. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins JA, Lee JC, Wilson SE. Susceptibility of intra-abdominal isolates at operation: a predictor of postoperative infection. Am Surg. 1993;59:791–796. [PubMed] [Google Scholar]
- 27.Koperna T, Schulz F. Prognosis and treatment of peritonitis. Arch Surg. 1996;131:180–186. [DOI] [PubMed] [Google Scholar]
- 28.Montravers P, Gauzit R, Muller C, et al. Emergence of antibiotic-resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy. Clin Infect Dis. 1996;23:486–494. [DOI] [PubMed] [Google Scholar]
- 29.Roehrborn A, Thomas L, Potreck O, et al. The microbiology of postoperative peritonitis. Clin Infect Dis. 2002;33:1513–1519. [DOI] [PubMed] [Google Scholar]