Abstract
Objective:
Lymphoscintigraphy for head and neck melanomas demonstrates a wide variation in lymphatic drainage pathways, and sentinel nodes (SNs) are reported in sites that are not clinically predicted (discordant). To assess the clinical relevance of these discordant node fields, the lymphoscintigrams of patients with head and neck melanomas were analyzed and correlated with the sites of metastatic nodal disease.
Methods:
In 362 patients with head and neck melanomas who underwent lymphoscintigraphy, the locations of the SNs were compared with the locations of the primary tumors. The SNs were removed and examined in 136 patients and an elective or therapeutic regional lymph node dissection was performed in 40 patients.
Results:
Lymphoscintigraphy identified a total of 918 SNs (mean 2.5 per patient). One or more SNs was located in a discordant site in 114 patients (31.5%). Lymph node metastases developed in 16 patients with nonoperated SNs, all underneath the tattoo spots on the skin used to mark the position of the SNs. In 14 patients SN biopsy revealed metastatic melanoma. After a negative SN biopsy procedure 11 patients developed regional lymph node metastases during follow-up. Elective and therapeutic neck dissections demonstrated 10 patients with nodal metastases, all located in predicted node fields. Of the 51 patients with involved lymph nodes, 7 had positive nodes in discordant sites (13.7%).
Conclusions:
Metastases from head and neck melanomas can occur in any SN demonstrated by lymphoscintigraphy. SNs in discordant as well as predicted node fields should be removed and examined to optimize the accuracy of staging.
Lymphoscintigrams demonstrated sentinel nodes in nonpredicted (discordant) sites in 114 of 362 head and neck melanoma patients (31.5%). Seven of 51 patients with regional node metastases (13.7%) had involvement in a discordant site. It is concluded that all sentinel nodes should be removed and examined to obtain reliable prognostic information.
Sentinel node (SN) biopsy procedures in the head and neck area are often technically demanding. The SNs may be small and are sometimes in sites that are not easily accessible, such as within the parotid gland or deep to the sternomastoid muscle. They may be located close to the primary site, making them difficult to identify following the injection of radioisotope at lymphoscintigraphy or blue dye at the time of surgery. Furthermore, in the head and neck area SNs are frequently found in multiple node fields, in contrast to melanomas located on extremities which usually drain to only 1 field.1,2 Despite these potential difficulties, 75–96% of head and neck SN biopsy procedures are reported to have been successful in studies using blue dye and/or radioisotopes.3–11 Moreover, complications after sentinel node biopsies in the head and neck area are reported to be very infrequent when performed by experienced surgeons.12
If the technique of selective SN biopsy is to provide accurate staging information, it is clearly essential that all SNs are identified and biopsied. However, SNs may be present in clinically unpredicted sites, reached by uncommon lymphatic drainage pathways. In patients with head and neck melanomas, previous studies have demonstrated that 34% to 84% of SNs are located in clinically unexpected (discordant) sites.1,2,13–16 These clinical predictions were based on the location of the primary lesions, and indicate the lymph node fields that surgeons would previously have dissected.17 Nevertheless, elective lymph node dissections of the clinically predicted fields proved to be effective in achieving local disease control in a retrospective study of 108 patients from our institution, with a low regional recurrence rate.18 It is perhaps relevant to note, however, that 3 of the 5 recurrences that occurred following these elective dissections were located outside the dissected field. Whether all sentinel nodes demonstrated by lymphoscintigraphy, including those in discordant sites, are clinically important has therefore been unclear.
To examine this issue, we analyzed a large sequential series of patients with head and neck melanomas who underwent lymphoscintigraphy at the Sydney Melanoma Unit (SMU) and correlated the location of their SNs with the location of their primary tumors. We also evaluated the clinical outcome for each patient with respect to metastatic involvement of lymph nodes, and compared this with the clinically predicted pattern of nodal metastasis.
METHODS
All patients with primary cutaneous head and neck melanomas treated at the SMU between 1986 and 2000 and who underwent preoperative lymphoscintigraphy were included in the study. Patient details, lymphoscintigram results, operation reports, pathology reports, and follow-up details were recorded using information contained in the SMU database. This database contains follow-up information recorded by SMU staff at the time of the follow-up visits and obtained from family doctors for some patients residing in remote places.
A total of 362 lymphoscintigrams were performed and interpreted by 1 of the authors (RFU) using previously reported techniques and criteria.1,19 Briefly, the radiopharmaceutical used was Technetium 99m antimony trisulfide colloid (99mTc-Sb2S3), which has a particle size of 5–40 nm. Multiple intradermal injections of 5 MBq, each in a volume of approximately 0.05 to 0.1 mL, were given around the melanoma excision-biopsy site. All studies were performed prior to definitive surgical treatment, which involved wide excision with or without SN removal or complete regional lymph node dissection. Scanning was performed using a rectangular field-of-view digital gamma camera with a low-energy, high-resolution collimator. Scanning was carried out immediately to identify the major lymphatic channels, followed by a delayed scan approximately 2.5 hours later. Anteroposterior and lateral views were obtained to localize the SNs, the positions of which were marked on the overlying skin with small tattoo spots made with a 21G needle and carbon black powder. A SN was defined as any lymph node that was observed to receive direct lymph drainage from the melanoma site. On the delayed images these nodes were usually the most intensely radioactive nodes on the scan.
The cervical lymph node levels at which the SNs were located were recorded using the standard terminology used in head and neck oncology. In this, level I represents the submandibular and submental nodes; level II the upper jugular, upper spinal accessory and jugulo-digastric nodes; level III the midjugular nodes with the lowest node represented by the jugulo-omohyoid node; level IV the lower jugular nodes including the medial supraclavicular nodes and level V the posterior triangle nodes.15 Other nodes identified during lymphoscintigraphy were postauricular nodes, occipital nodes, preauricular (parotid) nodes, axillary nodes, interval nodes and nodes located in the triangular intermuscular space on the back, lateral to the scapula.1,20,21
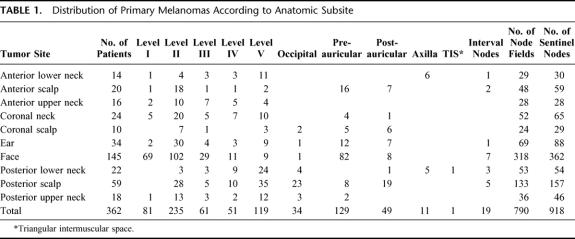
The location of each primary cutaneous melanoma was recorded on an anatomic map and subsequently matched to 1 of 10 anatomic subsites of the head and neck area, as previously described.15,22 The number of primary melanomas at each subsite is shown in Table 1. The clinical prediction of metastatic pathways for each primary melanoma was based on its anatomic site. The recommended elective lymph node dissections for each subsite have been described previously.17,22,23
TABLE 1. Distribution of Primary Melanomas According to Anatomic Subsite
Surgical management of the patients in this study varied with time because they were treated according to the protocols of several different clinical trials undertaken at the SMU over the 15-year study period. A total of 44 patients received part of their treatment in other hospitals and information from their medical records, apart from the lymphoscintigram results, was not included in the SMU database. Some patients were treated by wide excision only (n = 142), with the position of their SNs marked on the overlying skin for focused observation during clinical follow-up. Other patients underwent selective SN excision (n = 136) or elective (n = 33) or therapeutic (n = 7) complete regional lymph node dissection following wide excision of their primary lesion. When a SN biopsy was performed a standard, previously described technique was used.4 Four to 6 intradermal injections of Patent Blue V dye (Guerbert, Aulnay-Sous-Bois, France) were given around the primary excision-biopsy site, up to a volume of 1.0 to 2.0 mL. The blue dye was injected 10 to 15 minutes prior to surgery and exploration for the SNs was then performed at the site or sites marked on the overlying skin. In the early experience with SN biopsy procedures at the SMU, blue dye only was used for intraoperative SN location and identification, using information obtained from the preoperative lymphoscintigram to guide the surgical approach. From 1996, however, a hand-held gamma probe (Neoprobe 1000, Neoprobe Corporation, Dublin, OH) was used in conjunction with the blue dye mapping technique to assist with the identification of SNs by locating residual radioactivity in them following the preoperative lymphoscintigram.24 Elective lymph node dissections were performed according to the clinically predicted lymph node fields, as described by O'Brien et al.23 When preoperatively a lymph node was palpable or enlarged the procedure was recorded as a therapeutic lymph node dissection even if that node was not subsequently proven to contain metastatic melanoma. When no enlarged lymph nodes were palpable the procedure was recorded as an elective lymph node dissection.
Histopathological examination of SNs involved cutting them through their longitudinal axis in 3mm slices, and embedding all slices in paraffin. Four sequential 5-μ sections were cut from each paraffin block, the first and fourth stained with H&E and the second and third stained immunohistochemically for S100 protein and HMB45 respectively. The slides were examined by histopathologists experienced in the diagnosis and reporting of melanomas and SNs. In patients who developed recurrence of melanoma following a previously negative SLN, a single pathologist (RAS) reviewed the original slides and examined 6 further additional sections from each tissue block of all SLNs. The additional sections were stained with H&E and for S100 protein and HMB45 at each of 2 levels 50μ apart.
Non-SNs and all nodes in complete regional node dissection specimens were embedded whole in paraffin if <3mm in diameter (or sliced when >3 mm in diameter), and 5-μ hematoxylin and eosin-stained sections were examined microscopically for the presence of metastatic melanoma.
RESULTS
Lymphoscintigraphy
Table 1 shows the distribution of SNs in the various node fields. The figures in the columns indicate the number of patients who had a SN identified in each node field. The interval nodes were located on the posterior neck (n = 8), scalp (n = 5), cheek (n = 3), anterior to the ear (n = 2), and in the suprasternal notch (n = 1). In 1 patient (0.3%) no flow of tracer was demonstrated, 61 patients (16.9%) had a single SN, 133 patients (36.7%) had 2 SNs, 100 patients (27.6%) had 3 SNs, 49 patients (13.5%) had 4 SNs, 15 patients (4.1%) had 5 SNs, 2 patients (0.6%) had 6 SNs, and 1 patient (0.3%) had 8 SNs demonstrated on the lymphoscintigram. Many patients had drainage to multiple node fields and some patients had more than 1 SN in an individual node field. In 362 patients a total of 918 SNs were distributed through 790 fields; this represents an average of 2.5 nodes per patient and 1.2 SNs per lymph node field. SNs were identified in 122 sites that were discordant with clinical prediction. As an example of the observed variability, Figure 1 demonstrates the sites of SNs in 9 patients, each of whom presented with a melanoma on the right posterior lower neck.
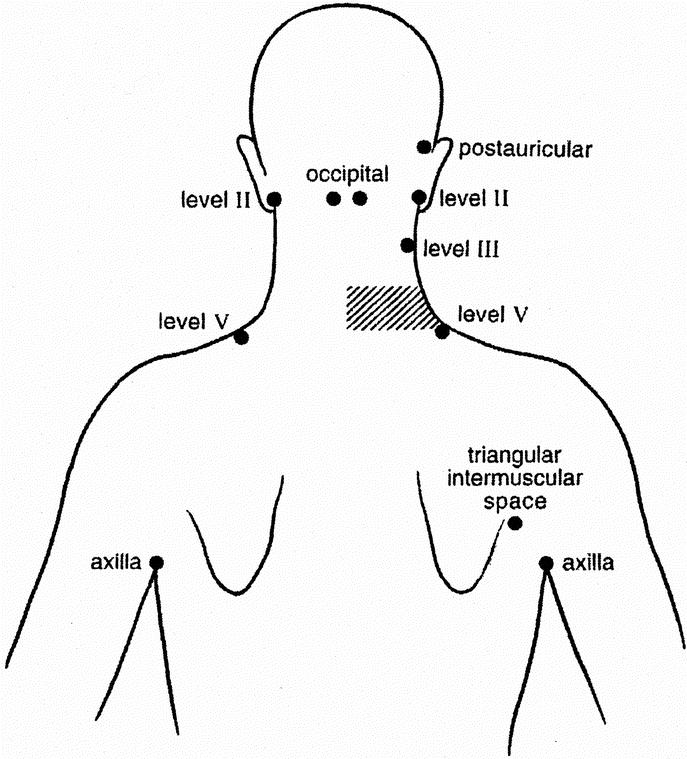
FIGURE 1. Location of sentinel nodes in 9 patients with primary cutaneous melanomas on the right lower posterior neck (shaded area).
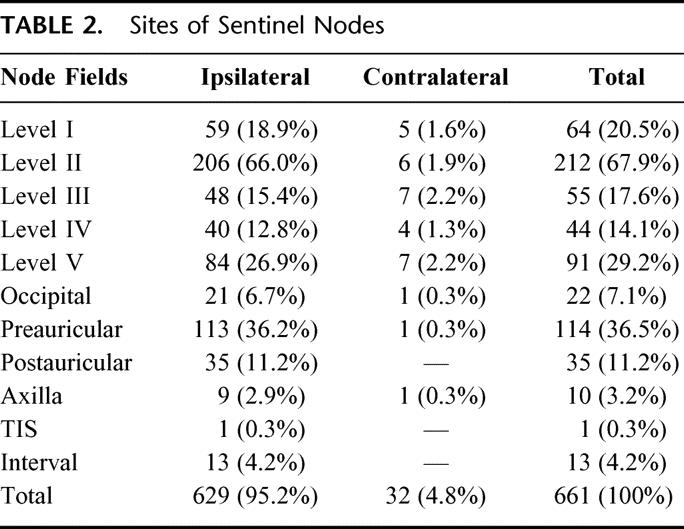
Of the total group of 362 patients, 50 had their primary melanomas located in or close to the midline of the head and neck region. Drainage to lymph node fields on both sides of the neck occurred in 24 (48%) of these patients; this could not be determined without a preoperative lymphoscintigram. In the remaining 312 patients who had their primary melanoma located clearly on the right or left side, 32 of 661 SNs (4.8%) were demonstrated in the contralateral head and neck area. Again, this situation in an individual patient could not be determined without a preoperative lymphoscintigram. Table 2 shows the location of all SNs according to their site in these 312 patients, and demonstrates that approximately 2 thirds of all head and neck melanoma patients have at least 1 SN in the ipsilateral neck at level II, regardless of the location of the primary.
TABLE 2. Sites of Sentinel Nodes
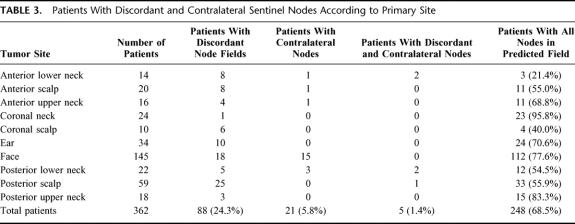
In some patients there was drainage from the primary tumor site to both ipsilateral and contralateral fields, and some had drainage to more than 1 ipsilateral, contralateral or otherwise discordant site. The total numbers of patients with SNs in these node fields are shown in Table 3. In this table midline primary melanomas are included but drainage to bilateral neck sites is not considered contralateral. A total of 114 patients (31.5%) were demonstrated to have 1 or more SNs located in clinically unpredicted sites. For the anterior and posterior lower neck and the anterior, coronal and posterior scalp, less than 60% of the SNs were in the predicted fields
TABLE 3. Patients With Discordant and Contralateral Sentinel Nodes According to Primary Site
Treatment
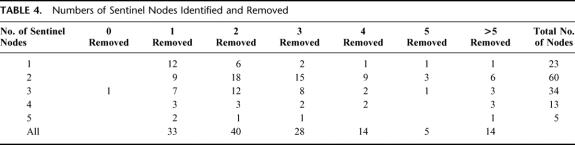
Figure 2 records the various treatments employed for the entire group of 362 SMU patients who had a preoperative lymphoscintigram. All patients underwent wide excision of the primary melanoma site. In 142 patients the locations of the SNs were simply marked on the skin with small tattoo spots for follow-up purposes. A SN biopsy procedure was performed in 136 patients. In all patients at least 1 node was removed, except in 1 patient in whom no SN could be identified. However, in 41 patients (30.4%) the number of harvested SNs was less than the number of SNs identified as such on the lymphoscintigram (Table 4). Prior to the introduction of the SN biopsy technique at the SMU, elective lymph node dissections were performed in 33 patients who were considered to be at particularly high risk of developing regional node recurrences. A therapeutic node dissection was performed in 7 patients in whom an enlarged node was palpable at the time of preoperative clinical examination.
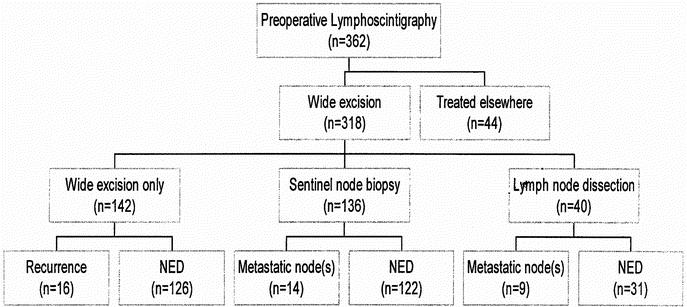
FIGURE 2. Patient treatment and outcomes after preoperative lymphoscintigraphy.
TABLE 4. Numbers of Sentinel Nodes Identified and Removed
Follow-up
Mean follow-up in the group of 142 patients treated by wide excision only was 24 months (range, 0–98 months). For 25 patients (17.6%) the follow-up period was less than 1 year and 17 patients (12%) were lost to follow-up. In 16 patients regional lymph node metastases were detected by clinical examination and/or ultrasound after a mean follow-up of 10 months (range, 2–32 months). The lymph node involved by metastatic melanoma was in every patient directly underneath the tattoo mark on the skin, which identified the site of an underlying SN. Therapeutic lymph node dissection was subsequently performed in 14 of these patients, and 2 patients were treated with radiotherapy only.
The mean follow-up for the group of 136 patients who underwent a selective SN biopsy procedure was 34 months (range, 0–79 months). 18 patients (13.2%) had a follow-up of less than 1 year and 2 (1.5%) were lost to follow-up. Metastatic involvement of at least 1 SN was demonstrated in 14 patients (10.3%). The sentinel node was the only positive lymph node after subsequent therapeutic lymph node dissection in 11 of these 14 patients. One patient had another positive node and 2 were treated with radiotherapy rather than a therapeutic lymph node dissection.
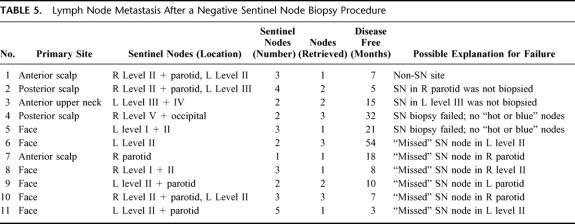
During follow-up, 11 of the 122 patients whose SNs had been reported as negative developed lymph node metastases in the head and neck area (9.2%; Table 5). Review of the original slides and examination of further sections from the tissue blocks both with H&E staining and with staining for S100 protein did not reveal a missed micrometastatic deposit in any of these patients. In 1 patient, a metastatic node was located in an area in the neck which had not been demonstrated to be the site of a SN on preoperative lymphoscintigraphy, but this patient had metastatic melanoma at other locations when the locoregional recurrence became apparent, making hematogenous dissemination rather than direct lymphatic spread a possibility. In 10 patients the metastatic lymph node was located in the same node field as that in which a SN had been demonstrated on their preoperative lymphoscintigram. In 2 of those patients the SN had not been biopsied at that particular site for specific reasons. Two patients had an unsuccessful SN biopsy according to the surgeon, with no hot or blue nodes being removed during the procedure. The other 6 patients had apparently successful SN biopsy procedures (with blue nodes retrieved), but all were performed in the period before the gamma probe was used intraoperatively. All these recurrences occurred in the parotid (n = 3) and in level II of the neck (n = 3).
TABLE 5. Lymph Node Metastasis After a Negative Sentinel Node Biopsy Procedure
Of those patients who had an elective lymph node dissection, 4 were found to have metastatic involvement of 1 or more lymph nodes (12.1%). Mean follow-up of the patients who underwent an elective or therapeutic lymph node dissection was 52 months (range, 6–151 months). Of the patients who underwent a therapeutic lymph node dissection, 5 of 7 had histologically confirmed metastatic involvement of the regional lymph nodes. In all elective and therapeutic dissections the previously identified SNs were within the dissected node fields.
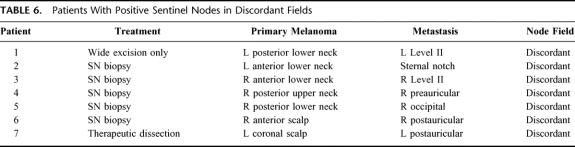
In the total group of 318 patients treated in our unit either with observation only or with some form of surgery, a total of 51 developed melanoma metastases in regional lymph nodes (16%). Of these patients with metastatic disease, 7 had a positive node located in a discordant field (13.7%; Table 6). The primary melanomas were located on the scalp in 2 of these patients, on the upper neck in 1, and on the lower neck in 4. All node metastases identified in discordant sites were ipsilateral, and metastatic involvement was not demonstrated in contralateral nodes in any patient.
TABLE 6. Patients With Positive Sentinel Nodes in Discordant Fields
DISCUSSION
The introduction of routine preoperative lymphoscintigraphy for patients with melanoma has provided new insights into lymphatic anatomy and physiology. Previous studies have demonstrated that cutaneous lymphatic drainage pathways are variable among patients, especially in the head and neck area.1,2,13–16 In the present study, clinically unpredicted lymphatic drainage to SNs in “discordant” sites was found in approximately one third of the patients, most notably from the lower neck and scalp. SNs receive lymph drainage directly from the primary tumor site and several studies have now confirmed the original proposal by Morton et al25 that these nodes are the nodes most likely to contain melanoma metastases.4,11,26,27 This pattern of dissemination first to SNs was confirmed in the present study, where 11 of 12 patients were found to have metastatic disease only in the biopsied SN and not in any lymph nodes in the subsequently performed full regional lymph node dissection. Moreover, all 16 patients who developed a regional metastasis during follow-up observation developed their metastatic node immediately beneath a tattoo spot marking the site of a SN. All SNs demonstrated by lymphoscintigraphy therefore seem to be as important and relevant for the evaluation of the regional lymph nodes in patients with melanomas in the head and neck region as they are for patients with primary melanomas on the trunk and limbs.
Several studies reported in the literature claim 90% or higher success rates for SN biopsy in patients with primary melanomas of the head and neck.3,5–7,9,10 The SN procedure has been reported to be even more successful when blue dye is used in combination with a gamma probe, rather than with blue dye only.5–7,10 In 2 multicenter studies, however, recovery rates for SNs in the head and neck area were 75% and 86%, respectively, which is considerably lower than it is for other regions of the body.8,11 In our study all but 1 of the patients had at least 1 node identified and removed during surgery, which could therefore be reported as a 99.3% success rate (ie, 135/136). However, the overall success rate of 70% for SN recovery compared with the number of nodes demonstrated by the preoperative lymphoscintigram was lower than all the above-mentioned studies. The present study represents the largest experience with head and neck SN biopsies reported to date, as far as we are aware, and the difference in the success rate therefore requires comment. First of all, despite previous reports in the literature, it is clear that SN biopsy in the head and neck area are often technically difficult. SNs can easily be missed on the preoperative lymphoscintigram when they are close to the primary tumor, due to radioactivity at the nearby or overlying injection site. During operation these underlying nodes can still be difficult to find due to residual radioactivity in the area and to blue staining of the subcutaneous tissues underlying the primary melanoma site. Also, SNs in the head and neck area and especially in the parotid region are often small (<5 mm in diameter), which makes them more difficult to identify.12 The major problem is that in the head and neck area more than 1 SN is often demonstrated. With our high quality lymphoscintigraphy using a small-particle colloid, and the use of dynamic as well as delayed images, more than 80% of the patients were demonstrated to have 2 or more SNs. The reported average number of 2.5 SNs per patients in this study is higher than the average number of SNs (1.25–2.3) reported by other investigators.3,5,6,9,10,12 If our lymphoscintigrams had been performed with a larger particle colloid (as routinely used in the United States), without meticulous observation of the early dynamic phase, it is likely that fewer SNs would have been identified, and the ‘success’ rate would therefore have been higher. Nevertheless in a number of patients some nodes reported as likely to be SNs were considered to be second tier nodes at the time of surgery, on the basis of blue dye staining, and were therefore not removed. Small particle colloids demonstrate more node fields per patient, and more SNs per field than large particle colloids, and are also more likely to demonstrate second tier nodes.1 However, the precise numbers of SNs can usually be determined with confidence based on early, dynamic imaging, ie, by careful assessment of lymphatic drainage during the first 10–15 minutes after tracer injection at the primary tumor site.
Another consideration is that, in their early experience, 2 of the 5 surgeons in our institution were not prepared to biopsy more than 2 different node fields and chose to biopsy only the brightest or hottest node or nodes when SNs were demonstrated in 3 or more node fields on the preoperative lymphoscintigram. Additionally, 1 surgeon was not prepared to biopsy nodes in the parotid, to avoid the theoretical risk of damage to facial nerve branches. And finally, in the early days of SN biopsy at the SMU, blue dye only was used instead of the combination of the gamma probe and blue dye used in recent years, which might also have contributed to the lower success rate.
In the present study 10.3% of SNs were found to contain metastatic melanoma deposits. This number is comparable to percentages of head and neck melanoma patients with positive nodes previously reported in the literature (9.4%-27%).3,5,6,8,9,12 However, 11 patients (9.2%) who were originally reported to be node negative after SN biopsy, developed melanoma metastases in regional node fields. In 6 patients the metastases occurred in a lymph node area in which a SN had been demonstrated by preoperative lymphoscintigraphy and where a blue node was retrieved during operation. These false negative cases could have resulted from surgical failure (wrong node removed) or could have been due to inadequate histologic assessment of the removed SN. Bostick et al have demonstrated that micrometastases not shown with H&E staining or immunohistochemistry can sometimes be demonstrated using PCR techniques.28 We reviewed all histology slides and further sections cut from the tissue blocks and could not demonstrate missed metastatic deposits in the nodes that had been removed, but we did not use PCR to assess whether these nodes contained micrometastatic disease. In 1 patient metastatic disease developed in a nonsentinel node site, but this was associated with distant and other locoregional disease, making it possible that hematogenous spread could explain the involvement of these nodes and not the SNs.
SNs were demonstrated in discordant fields in 31.5% of patients in this study, and lymph node metastases were found in discordant fields in 13.7% of patients who developed lymph node metastases. As mentioned previously, in this series of patients with head and neck melanomas there was an average of 2.5 sentinel nodes per patient, and in the great majority of them only 1 of the sentinel nodes contained metastatic cells. If SN metastasis is as likely to occur in a discordant site as it is in a clinically predicted site, the calculated chance of a positive node occurring in a discordant field is therefore 12.6% (ie, 31.5/2.5), which is remarkably close to the percentage actually found in this study (13.7%). Roozendaal et al29 recently reported that SNs outside the predicted node field can contain metastases, but this study involved all primary cutaneous melanoma sites. A previous study from our own institution demonstrated discordant nodes in 13 of 169 patients (7.7%) who underwent therapeutic lymph node dissections for metastatic head and neck melanoma.30 Not all these patients had preoperative lymphoscintigraphy, so information indicating whether these metastatic nodes were SNs or non-SNs was lacking. Contralateral metastases were not demonstrated in that study, nor in the present study, with all predicted and discordant nodes containing melanoma metastases located on the same side as the primary tumor. After completion of data collection for the present report, however, a 19-year-old man was referred for treatment at the SMU following biopsy of a 1.2-mm thick melanoma on his lower left cheek. Lymphoscintigraphy (Fig. 3) revealed drainage to 2 SNs, 1 in level II of the ipsilateral neck, and 1 in level IV of the contralateral neck. When removed, both SNs were found to contain micrometastatic melanoma.
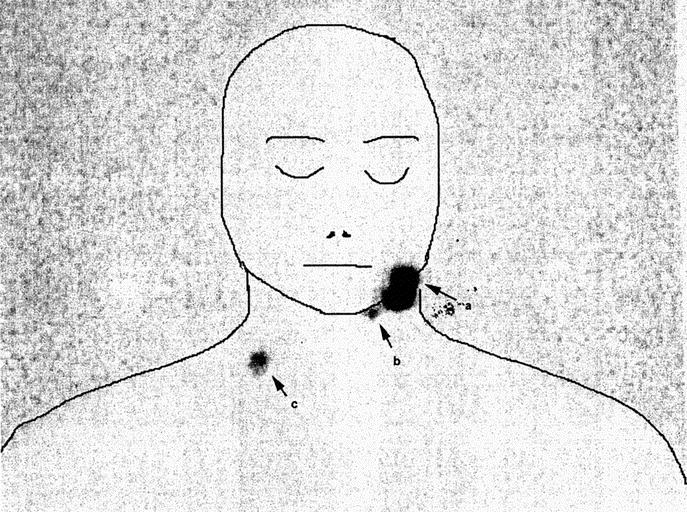
FIGURE 3. Lymphoscintigram of a 19-year-old man with a primary melanoma on the lower left cheek (a), demonstrating sentinel nodes in level II of the ipsilateral (left) neck (b) and in level IV of the contralateral (right) neck(c). Micrometastatic melanoma was identified in the sentinel node in the lower right neck as well as in the sentinel node in the upper left neck.
It is interesting to note that for melanomas on the anterior and posterior lower neck and on the anterior, coronal and posterior scalp, more than 40% of SNs were in discordant locations. This finding should alert the clinician to search with particular care for possible SNs in nonstandard sites when primary tumors are located in these areas.
The results of the present study of patients with primary cutaneous head and neck melanomas show that one third of SNs demonstrated by lymphoscintigraphy are located in discordant field sites. Since all SNs, including those in discordant sites, might contain metastatic melanoma, accurate staging for melanoma patients can only be achieved by removal and examination of all SNs, regardless of their location. To identify all SNs, high quality preoperative lymphoscintigraphy is considered to be essential.
Footnotes
Supported by the Melanoma Foundation of the University of Sydney. V. S. K. Ka was a research student supported by a Fullbright Scholarship. J. H. W. de Wilt was a fellow in surgical oncology supported by a grant from the Dutch Cancer Society, and is currently working at the Department of Surgical Oncology at the Erasmus MC Rotterdam Daniel den Hoed Cancer Center, Rotterdam, The Netherlands.
Reprints: J. F. Thompson, MD, Sydney Melanoma Unit, Royal Prince Alfred Hospital, Camperdown, New South Wales 2050, Australia. Email: thompson@smu.org.au.
REFERENCES
- 1.Uren RF, Thompson JF, Howman-Giles RB. Lymphatic drainage of the skin and breast. Locating the sentinel nodes. Amsterdam: Harwood Academic Publishers; 1999. [Google Scholar]
- 2.Thompson JF, Uren RF, Shaw HM, et al. Location of sentinel lymph nodes in patients with cutaneous melanoma: new insights into lymphatic anatomy. J Am Coll Surg. 1999;189:195–204. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Foshag LJ, et al. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. J Clin Oncol. 1993;11:1751–1756. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res. 1995;5:255–260. [DOI] [PubMed] [Google Scholar]
- 5.Bostick P, Essner R, Sarantou T, et al. Intraoperative lymphatic mapping for early-stage melanoma of the head and neck. Am J Surg. 1997;174:536–539. [DOI] [PubMed] [Google Scholar]
- 6.Wells KE, Rapaport DP, Cruse CW, et al. Sentinel lymph node biopsy in melanoma of the head and neck. Plast Reconstr Surg. 1997;100:591–594. [DOI] [PubMed] [Google Scholar]
- 7.Alex JC, Krag DN, Harlow SP, et al. Localization of regional lymph nodes in melanomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;24:135–140. [DOI] [PubMed] [Google Scholar]
- 8.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Ann Surg. 1999;230:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen L, Koops HS, Nieweg OE, et al. Sentinel node biopsy for melanoma in the head and neck region. Head Neck. 2000;22:27–33. [DOI] [PubMed] [Google Scholar]
- 10.Carlson GW, Murray DR, Greenlee R, et al. Management of malignant melanoma of the head and neck using dynamic lymphoscintigraphy and gamma probe-guided sentinel lymph node biopsy. Arch Otolaryngol Head Neck Surg. 2000;126:433–437. [DOI] [PubMed] [Google Scholar]
- 11.Cascinelli N, Belli F, Santinami M, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann Surg Oncol. 2000;7:469–474. [DOI] [PubMed] [Google Scholar]
- 12.Ollila DW, Foshag LJ, Essner R, et al. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol. 1999;6:150–154. [DOI] [PubMed] [Google Scholar]
- 13.Eberbach MA, Wahl RL, Argenta LC, et al. Utility of lymphoscintigraphy in directing surgical therapy for melanomas of the head, neck, and upper thorax. Surgery. 1987;102:433–442. [PubMed] [Google Scholar]
- 14.Wells KE, Cruse CW, Daniels S, et al. The use of lymphoscintigraphy in melanoma of the head and neck. Plast Reconstr Surg. 1994;93:757–761. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien CJ, Uren RF, Thompson JF, et al. Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg. 1995;170:461–466. [DOI] [PubMed] [Google Scholar]
- 16.Leong SP, Achtem TA, Habib FA, et al. Discordancy between clinical predictions vs lymphoscintigraphic and intraoperative mapping of sentinel lymph node drainage of primary melanoma. Arch Dermatol. 1999;135:1472–1476. [DOI] [PubMed] [Google Scholar]
- 17.Shah JP, Kraus DH, Dubner S, et al. Patterns of regional lymph node metastases from cutaneous melanomas of the head and neck. Am J Surg. 1991;162:320–323. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien CJ, Petersen-Schaefer K, Ruark D, et al. Radical, modified, and selective neck dissection for cutaneous malignant melanoma. Head Neck. 1995;17:232–241. [DOI] [PubMed] [Google Scholar]
- 19.Uren RF, Howman-Giles RB, Shaw HM, et al. Lymphoscintigraphy in high-risk melanoma of the trunk: predicting draining node groups, defining lymphatic channels and locating the sentinel node. J Nucl Med. 1993;34:1435–1440. [PubMed] [Google Scholar]
- 20.Uren RF, Howman-Giles R, Thompson JF, et al. Lymphatic drainage to triangular intermuscular space lymph nodes in melanoma on the back. J Nucl Med. 1996;37:964–966. [PubMed] [Google Scholar]
- 21.Uren RF, Howman-Giles R, Thompson JF, et al. Interval nodes: the forgotten sentinel nodes in patients with melanoma. Arch Surg. 2000;135:1168–1172. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien CJ, Gianoutsos MP, Morgan MJ. Neck dissection for cutaneous malignant melanoma. World J Surg. 1992;16:222–226. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien CJ, Shah JP, Alfonsus JMB. Neck dissection and parotidectomy for melanoma. In: Thompson JF, Morton DL, Kroon BBR, eds. Textbook of melanoma. London: Martin Dunitz Publishers; 2004; p. 296–306. [Google Scholar]
- 24.Thompson JF, Niewind P, Uren RF, et al. Single-dose isotope injection for both preoperative lymphoscintigraphy and intraoperative sentinel lymph node identification in melanoma patients. Melanoma Res. 1997;6:500–506. [DOI] [PubMed] [Google Scholar]
- 25.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 26.Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16:2253–2260. [DOI] [PubMed] [Google Scholar]
- 28.Bostick PJ, Morton DL, Turner RR, et al. Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase-polymerase chain reaction in early-stage melanoma patients. J Clin Oncol. 1999;17:3238–3244. [DOI] [PubMed] [Google Scholar]
- 29.Roozendaal GK, de Vries JD, van Poll D, et al. Sentinel nodes outside lymph node basins in patients with melanoma. Br J Surg. 2001;88:305–308. [DOI] [PubMed] [Google Scholar]
- 30.Pathak I, O'Brien CJ, Petersen-Schaeffer K, et al. Do nodal metastases from cutaneous melanoma of the head and neck follow a clinically predictable pattern? Head Neck. 2001;23:785–790. [DOI] [PubMed] [Google Scholar]