Figure 5.
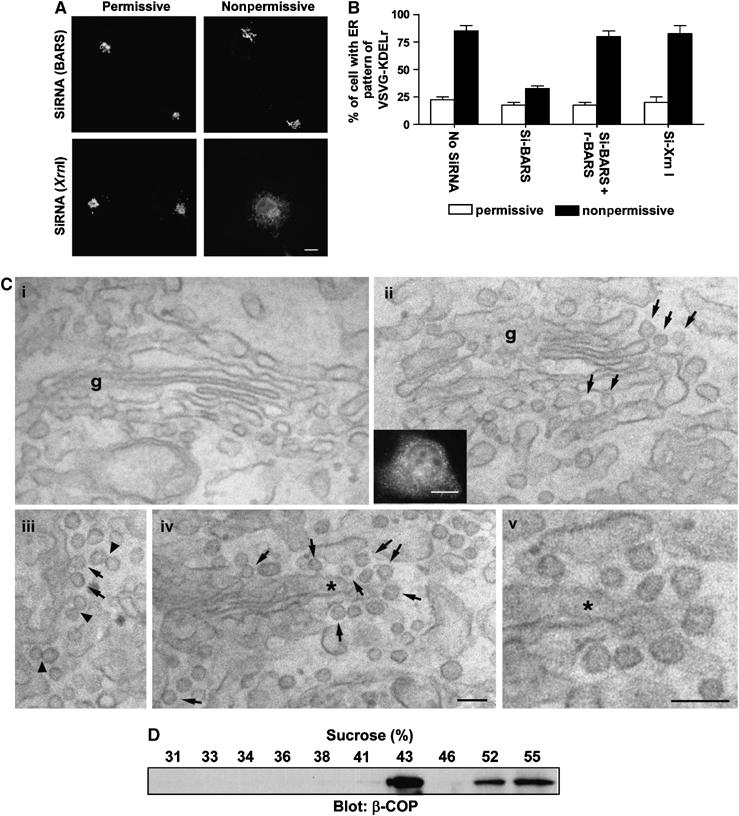
In vivo confirmation that BARS is critical for fission of COPI vesicles. (A) Knocking down BARS inhibits the retrograde transport of a chimeric KDELR. COS cells were transfected with VSVG-ts045-KDELR-myc, and siRNA against either BARS or Xrn1 (an exoribonuclease as control). Cells were then examined for the distribution of the chimeric KDELR by immunofluorescence microscopy upon shift to the nonpermissive temperature for 1 h. Representative images are shown (bar, 10 μm). (B) Ectopic expression of rat BARS restores retrograde transport of a chimeric KDELR that had been inhibited by siRNA directed against monkey BARS. The same experiment in (A) was performed, except for the additional condition of ectopically expressing rat BARS in COS cells. The mean with standard error was obtained from three independent experiments. (C) The CTP mutant induces increased coated buds in Golgi areas of transfected cells. COS cells were either mock transfected (i) or transfected with the CTP mutant (ii–v). Correlative light-EM technique was then used. A representative transfected cell is examined at both the light level (inset in ii) and at the EM level (ii–v). Note the presence of many 50–80 nm buds (arrows) with visible coating and narrow ‘necks' in CTP-transfected cells, suggesting that they are coated buds. EM tomography (not shown) was also performed on 200 nm sections to confirm that most of these round profiles were buds. Arrowheads indicate double vesicles/buds with an electron-dense connection, g (Golgi complex). The asterisk in (iv) indicates the region enlarged and shown in (v); bar, 100 nm for EM images and 10 μm for light microscopy image. (D) The CTP-induced buds have similar buoyant density as reconstituted COPI vesicles. CHO Golgi membrane washed with 0.5 M KCl was used for the two-stage incubation system, with GAP and CTP added at the second stage. After incubation, the reaction was subjected to high-salt treatment and pipette-induced shearing, followed by centrifugation to release buds into the supernatant fraction. This fraction was then analyzed by equilibrium centrifugation, followed by immunoblotting of gradient fractions with β-COP.
