Figure 7.
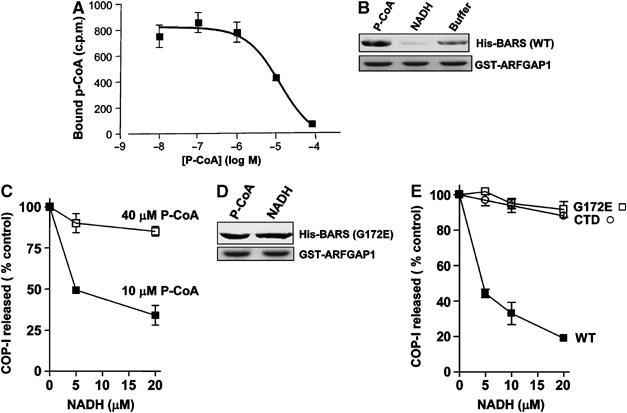
The interaction of BARS and GAP is oppositely regulated by p-coA and NADH, which act as cofactors to affect vesicle formation. (A) Binding to p-coA by BARS. BARS was incubated with labeled p-coA and increasing concentrations of unlabeled p-coA as indicated. The amount of radiolabeled p-coA that remained bound was then quantified. The mean derived from three independent experiments is shown with standard error. (B) The interaction between BARS and GAP is enhanced in the presence of p-coA and reduced in the presence of NADH. Soluble BARS was preincubated with either p-coA or NADH, followed by incubation with GAP on beads as GST fusion protein for a pulldown assay. Beads were then immunoblotted for BARS (upper panel) and Coomassie-stained for GST-GAP (lower panel). (C) NADH inhibits the release of COPI from Golgi membrane in the vesicle reconstitution assay, and this inhibition is reversed by titrating in increasing levels of p-coA. The two-stage incubation system was performed using Golgi membrane washed with 3 M KCl. After the second-stage incubation that used GAP and BARS and levels of p-coA and/or NADH as indicated, the level of β-COP released into the supernatant was quantified and then normalized to control, which is derived from the condition that used only GAP and BARS without adding either p-coA or NADH in the incubation. The mean of this normalized value derived from three independent experiments is shown with standard error. (D) NADH does not inhibit binding to GAP by the G172E point mutant of BARS. Soluble G172E mutant BARS was preincubated with either p-coA or NADH, followed by incubation with GAP on beads as GST fusion protein for a pulldown assay. Beads were then immunoblotted for BARS (upper panel) and Coomassie-stained for GST-GAP (lower panel). (E) NADH does not inhibit the release of coatomer from Golgi membrane during the second-stage incubation using either the G172E point mutant or the CTD mutant of BARS. The same assay was used as described in (C), with only increasing level of NADH present as indicated.
