Figure 4.
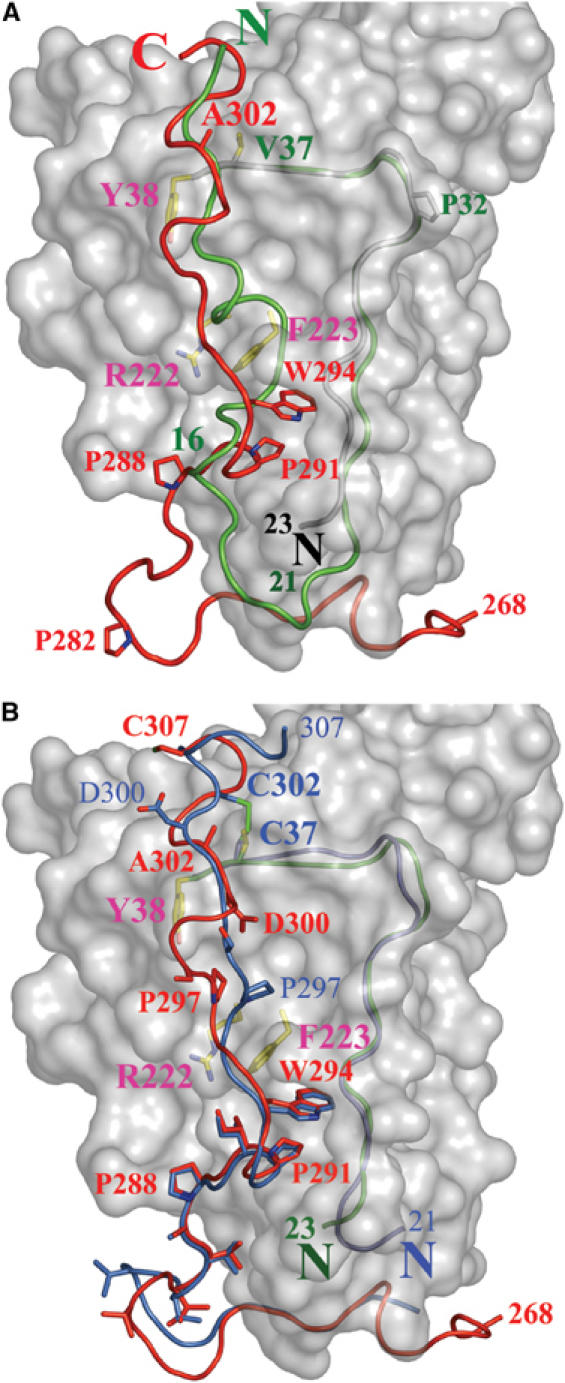
Superimposition of gD(23–306)307C and gD285 from the gD285–HVEM complex structure. (A) The gD(23–306)307C core is represented as a white surface. The N-terminal residues of gD285 (1–16, in green) from the gD285–HVEM complex occupy the same space as residues from the C-terminus of gD(23–306)307C (289–307, in red). The side chains of Tyr38, Phe223, and Arg222, three residues involved in nectin-1 binding and buried under C-terminal residues in gD(23–306)307C, are shown. Val37 and Ala302, two residues predicted to form a disulfide if mutated in cysteines, are also shown. (B) The C-terminus of gD(23–306)307C (in red) and of gD316V37C–A302C (in blue) occupy identical positions in the 285–296 region, but adopt different conformations in the 297–307 region. The disulfide bond between Cys302 and Cys37, the side chains of several C-terminal residues as well as those of Tyr38, Phe223, and Arg222 are shown. The N-terminal residues of gD(23–306)307C and gD316V37C–A302C are colored in green and blue, respectively.
