Figure 2.
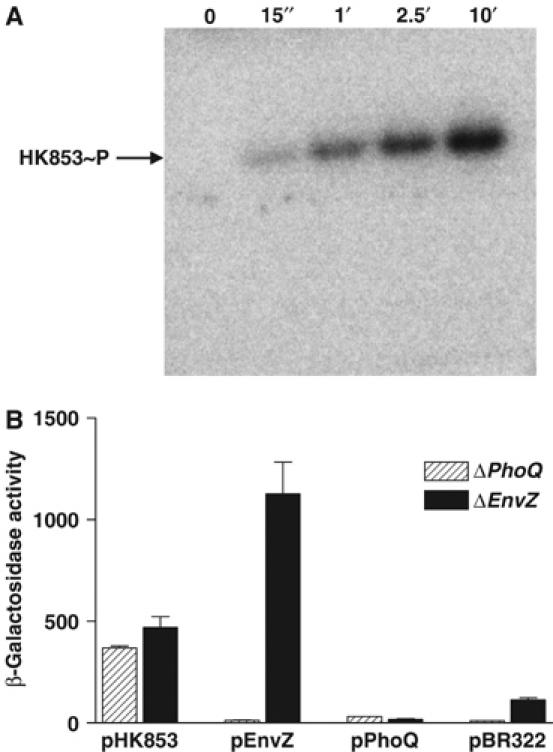
In vitro and in vivo HK853 activity. (A) Time course of in vitro autophosphorylation of HK853–CD with [γ-32P]ATP. In all, 1–2 μM of HK853–CD was incubated in reaction buffer and samples were removed at indicated time points, reaction stopped by addition of SDS–PAGE sample buffer, subjected to gel electrophoresis, and phosphorylated protein was visualized by phosphorimaging. (B) Activation of the PhoQ–PhoP and EnvZ–OmpR HK–RR systems. E. coli reporter strains containing a PhoP-activated lacZ, but devoid of PhoQ (ΔPhoQ), or an OmpR-activated lacZ, but devoid of EnvZ (ΔEnvZ), were transformed with the pBR322-derived plasmids pHK853, pEnvZ, pPhoQ or pBR322 (expressing the respective full-length sensor kinases or a negative control). Response was assayed by β-galactosidase activity. The low activation by PhoQ was due to repressing divalent cations in culture media.
