Abstract
The meiotic recombination checkpoint, which is triggered by defects in recombination or chromosome synapsis, arrests sporulating cells of Saccharomyces cerevisiae at pachytene by preventing accumulation of active Clb-Cdc28. We compared the effects of manipulating the three known targets of the meiotic recombination checkpoint, NDT80, SWE1, and SUM1, in dmc1-arrested cells. Ndt80 is an activator of a set of middle sporulation-specific genes (MSGs), which includes CLB genes and genes involved in spore wall formation; Swe1 inhibits Clb-Cdc28 activity; and Sum1 is a repressor of NDT80 and some MSGs. Activation of the checkpoint leads to inhibition of Ndt80 activity and to stabilization of Swe1 and Sum1. Thus, dmc1-arrested cells fail to express MSGs, arrest at pachytene, and do not form spores. Our study shows that dmc1/dmc1 sum1/sum1 cells expressed MSGs prematurely and at high levels, entered the meiotic divisions efficiently, and in some cases formed asci containing mature spores. In contrast, dmc1/dmc1 swe1/swe1 cells expressed MSGs at a very low level, were inefficient and delayed in entry into the meiotic divisions, and never formed mature spores. We found that cells of dmc1/dmc1 sum1/sum1 ndt80/ndt80 and dmc1/dmc1 swe1/swe1 ndt80/ndt80 strains arrested at pachytene and that dmc1/dmc1 or dmc1/dmc1 swe1/swe1 cells overexpressing NDT80 were less efficient in bypassing checkpoint-mediated arrest than dmc1/dmc1 sum1/sum1 cells. Our results are consistent with previous suggestions that increased Clb-Cdc28 activity, caused by mutation of SWE1 or by an NDT80-dependent increase in CLB expression, allows dmc1/dmc1 cells to exit pachytene and that subsequent upregulation of Ndt80 activity by a feedback mechanism promotes entry into the meiotic divisions. Spore morphogenesis, however, requires efficient and timely activation of MSGs, which we speculate was achieved in dmc1/dmc1 sum1/sum1 cells by premature expression of NDT80.
Propagation of viable cells is ensured by surveillance mechanisms that monitor successful completion of key events at various points throughout the cell cycle, referred to as checkpoints (16). If a defect is sensed, a signal transduction pathway becomes active, leading to an arrest or delay in the cell cycle so that the defect can be corrected. Yeast has proven to be a useful organism to study how a checkpoint signal is established, the mechanisms that detect and relay these signals to downstream targets, and the manner by which these targets lead to cell-cycle arrest (reviewed in references 9, 11, 28, and 33). As cells of Saccharomyces cerevisiae progress through the mitotic cell cycle, checkpoint mechanisms monitor such events as DNA replication, spindle assembly, bud morphogenesis, and nuclear migration. Defects in these events, as well as externally promoted perturbations, such as DNA damage, trigger checkpoint-mediated cell-cycle arrest. An additional checkpoint, the meiotic recombination checkpoint, operates during meiosis in response to defects in meiotic recombination and chromosome synapsis and arrests cells at pachytene, prior to entry into the meiotic divisions (reviewed in reference 38). This checkpoint, which shares components with the DNA damage checkpoint, prevents cells from attempting to segregate chromosomes under conditions that would lead to chromosome nondisjunction and spore inviability.
MATa/MATα cells of S. cerevisiae enter meiosis when starved for nitrogen in the presence of a nonfermentable carbon source. On completion of premeiotic DNA replication, the cells enter a relatively lengthy prophase, during which chromosomes undergo a high level of recombination and are brought into close juxtaposition by formation of the proteinaceous synaptonemal complex (SC) along the lengths of paired chromosomes (reviewed in reference 54). The pachytene stage of prophase is characterized by fully formed tripartite SCs and the presence of duplicated but unseparated spindle pole bodies. During pachytene, recombination intermediates are resolved and the cells become committed to undergoing the meiotic divisions. Exit from pachytene requires Cdc28 activity (41, 52), which is promoted by accumulation of Clb1 and to a lesser extent Clb3 and Clb4 (10, 14). As cells exit pachytene, the SC dissolves, chiasmata appear, and the duplicated spindle pole bodies migrate to generate the meiosis I spindle. The two meiotic divisions, first the reductional division in which homologous chromosomes separate and then the equational division in which sister chromatids separate, occur in quick succession. Both mitosis and meiosis in yeast occur in the absence of breakdown of the nuclear envelope. On completion of the meiotic divisions, the nucleus has a four-lobed shape, with each lobe containing a haploid complement of chromosomes. It is the formation of the prospore membrane that finally encloses and separates the meiotic products into nuclear compartments. Prospore membrane formation initiates by the fusion of vesicles at the modified outer plaque of the spindle pole bodies (21; reviewed in references 23 and 34). The prospore membrane then expands and engulfs each nuclear lobe within a double membrane. Deposition of spore wall material within the prospore membrane generates the four layers of the mature spore wall. The two electron-lucent innermost layers resemble the vegetative cell wall and are rich in glucan and mannan (reviewed in reference 42). The two outer layers, which are spore specific, consist of a diffuse chitosan-containing layer and a thin, electron-dense outermost layer that contains a dityrosine cross-linked molecule (reviewed in reference 42). The final product of sporulation is a tetrahedrally shaped ascus that contains four haploid gametes encased in spore walls. This program of genetic and morphological events depends on the sequential expression of at least four subsets of sporulation-specific genes, the early, middle, mid-late, and late genes (8, 36; reviewed in reference 23).
The meiotic recombination checkpoint, which is activated in response to defects in recombination or SC formation, arrests cells at pachytene. Such defects are caused by mutations in genes such as DMC1 (4), which encodes a strand exchange enzyme required for meiotic recombination, and ZIP1, which encodes an integral component of the SC (45). Checkpoint-mediated pachytene arrest can be alleviated by mutations in genes that are required to sense the defect and transmit the checkpoint signal, such as RAD17, RAD24, MEC1, DDC1, and MEC3 (2, 19, 32, 39). This arrest can also be bypassed by mutations in genes encoding meiotic chromosomal proteins that may regulate or generate the arrest signal, such as MEK1 and RED1 (2, 3, 50, 53). A third way that checkpoint-mediated pachytene arrest can be alleviated is by mutation of genes encoding chromatin-silencing factors, such as SIR2, PCH2, and DOT1 (39, 40). Mutation of any one of these checkpoint genes allows cells with defects in recombination or SC formation to enter the meiotic divisions.
Ndt80 (7, 18, 49), Swe1 (27), and Sum1 (29) have been shown to be targets of the meiotic recombination checkpoint. Ndt80, which is a global activator of a large set of middle sporulation-specific genes including CLB1, binds to the mid- sporulation element (MSE) that is present in the promoter region of many of these genes (7, 8, 18). As initially suggested by Xu and colleagues (52), Ndt80 controls entry into the meiotic divisions by regulating the activity of Cdc28 kinase. This is accomplished indirectly through Ndt80-dependent activation of CLB gene expression during sporulation (7, 18). In checkpoint-arrested cells, Ndt80 is inactivated and middle sporulation genes are not expressed (7, 18). The activity of Ndt80 may depend on phosphorylation; Tung and coworkers (49) have shown that Ndt80 is phosphorylated during sporulation in wild-type cells but not in cells subject to checkpoint-mediated pachytene arrest. Overexpression of NDT80 in zip1/zip1 cells promotes its phosphorylation and allows these cells, which would normally respond to the meiotic recombination checkpoint, to ignore the pachytene arrest signal (49).
Sum1, a DNA-binding transcriptional repressor of NDT80, SMK1, and other genes, has been shown to recognize the same site as Ndt80 (51). SUM1 was originally identified on the basis of its dominant suppressor allele, SUM1-1, which restores silencing function to cells mutated for various SIR genes (6, 20, 24, 30). More recently, SUM1 was identified as contributing to mitotic repression of various middle sporulation-specific genes (51). Significantly, Lindgren et al. (29) found that mutation of SUM1 alleviates pachytene arrest in dmc1/dmc1 cells. In contrast to our study (see below), these investigators found that expression of middle sporulation-specific genes was not restored in sum1/sum1 dmc1/dmc1 cells (29). The amount of Sum1 protein decreases transiently in wild-type cells midway through sporulation (29). This may be a regulated event that is required for cells to progress into the meiotic divisions, as Sum1 is stabilized in dmc1/dmc1 cells but not in dmc1/dmc1 rad17/rad17 cells, which bypass the pachytene arrest signal (29).
SWE1, the third known target of the meiotic recombination checkpoint, encodes a kinase that inhibits the activity of Cdc28 by phosphorylation of Y19 (5). Unlike the case in Schizosaccharomyces pombe, inhibitory phosphorylation of Cdc28 is not required in S. cerevisiae for the mitotic cell-cycle arrest that is mediated by the DNA replication or DNA damage checkpoints (1, 43). However, in S. cerevisiae, the bud morphogenesis checkpoint (reviewed in reference 28) and the meiotic recombination checkpoint (27) depend on Swe1-mediated phosphorylation of Cdc28. Swe1 has been found to be more abundant and to be hyperphosphorylated in cells arrested at pachytene (27).
Various studies have indicated that different yeast strains have different responses to defects that activate the meiotic recombination checkpoint. For example, mutation of DMC1 leads to permanent arrest at pachytene in SK1-derived strains (4) but only delays entry into the meiotic divisions in BR2495-derived strains (37). Mutation of ZIP1 leads to complete arrest in BR2495-derived strains (48), but not in SK1-derived (44, 46) and other (27) strains. Mutation of GIP1, which encodes a sporulation-specific targeting subunit for the type I protein phosphatase Glc7, leads to arrest in pachytene in BR2495-derived strains (2), whereas SK1-derived gip1/gip1 strains complete the meiotic divisions but are defective in spore wall formation (47). The reasons for these strain-specific differences in arrest phenotypes are not clear, but their existence indicates that detailed comparisons of the effects of mutations on progression through sporulation must be made within the same strain background. In this study we have compared the roles of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint in cells of the rapidly sporulating SK-1 strain. We have monitored three aspects of the sporulation program: the ability of the cells to enter the meiotic divisions; the pattern of sporulation-specific gene expression at the time that cells normally exit pachytene and undergo the meiotic divisions; and the formation of mature spores. Our results suggest that increasing Clb-Cdc28 activity in cells responding to the meiotic recombination checkpoint suffices to drive these cells into the meiotic divisions. Spore formation in these cells, however, requires timely expression of middle sporulation-specific genes.
MATERIALS AND METHODS
Yeast strains and genetic procedures.
Table 1 lists the S. cerevisiae strains used in this study. Strain JPY57 was obtained by mating strains JPY47 and JPY50, which were constructed as follows. The diploid obtained by mating DKB407 and DKB408 was transformed with a PCR product containing the kanMX6 cassette flanked by sequences from upstream and downstream of the SWE1 open reading frame. The resultant strain was sporulated, and random MATa Δswe1::kanMX6 and MATα Δswe1::kanMX6 spore segregants were identified (31) and verified by PCR analysis. Strain JPY225 was obtained by mating random MATa Δdmc1::ARG4 Δsum1::kanMX6 and MATα Δdmc1::ARG4 Δsum1::kanMX6 spore segregants of the diploid obtained by mating DKB625 and JPY141. Strain JPY65 was obtained by mating random MATa Δdmc1::ARG4 Δswe1::kanMX6 and MATα Δdmc1::ARG4 Δswe1::kanMX6 spore segregants of the diploid obtained by mating DKB626 and JPY47. Strain JPY173 was constructed by first mating the haploid MATa Δdmc1::ARG4 Δswe1::kanMX6 strain used to construct JPY65 with the strain NKY2296B; the resultant strain was sporulated and random MATa Δdmc1::ARG4 Δswe1::kanMX6 Δndt80::LEU2 and MATα Δdmc1::ARG4 Δswe1::kanMX6 Δndt80::LEU2 spore segregants were identified and mated. JPY232 was constructed by first mating the haploid MATα Δdmc1::ARG4 Δsum1::kanMX6 strain used to construct JPY225 and the haploid MATa Δsum1::kanMX6 Δndt80::LEU2 strain used to construct JPY215 (35); the resultant strain was sporulated and random MATa Δdmc1::ARG4 Δsum1::kanMX6 Δndt80::LEU2 and MATα Δdmc1::ARG4 Δsum1::kanMX6 Δndt80::LEU2 spore segregants were identified and mated.
TABLE 1.
S. cerevisiae strains used in this study
| SK1-derived strain | Genotype | Source |
|---|---|---|
| DKB407 | MATα ho::LYS2 lys2 ura3 leu2 his4X-ADE2-his4B ade2 | D. Bishop (32) |
| DKB408 | MATaho::LYS2 lys2 ura3 leu2 his4X-ADE2-his4B ade2 | D. Bishop (32) |
| DKB98 | MATa/MATα lys2/lys2 ho::LYS2/ho::LYS2 ura3/ura3 leu2::hisG/leu2::hisG his4X::LEU2/his4B::LEU2 arg4-NspI/arg4-BglII | D. Bishop (32) |
| NKY2296 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 Δndt80::LEU2/Δndt80::LEU2 | N. Kleckner (52) |
| NKY2296B | MATα ho::LYS2 lys2 ura3 leu2 Δndt80::LEU2 | This laboratory (18) |
| JPY141 | MATα ho::LYS2 lys2 ura3 leu2 his4X-ADE2-his4B ade2 Δsum1::kanMX6 | (35) |
| JPY214 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 his4X-ADE2-his4B/his4X-ADE2-his4B ade2/ade2 Δsum1::kanMX6/Δsum1::kanMX6 | (35) |
| DKB625 | MATaho::LYS2 lys2 ura3 leu2::hisG arg4-NspI Δdmc1::ARG4 | D. Bishop (32) |
| DKB626 | MATα ho::LYS2 lys2 ura3 leu2::hisG arg4-NspI Δdmc1::ARG4 | D. Bishop (32) |
| DKB608 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2::hisG/leu2::hisG arg4-NspI/arg4-NspI Δdmc1::ARG4/Δdmc1::ARG4 | D. Bishop (32) |
| JPY47 | MATaho::LYS2 lys2 ura3 leu2 his4X-ADE2-his4B ade2 Δswe1::kanMX6 | This study |
| JPY50 | MATα ho::LYS2 lys2 ura3 leu2 his4X-ADE2-his4B ade2 Δswe1::kanMX6 | This study |
| JPY57 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 his4X-ADE2-his4B/his4X-ADE2-his4B ade2/ade2 Δswe1::kanMX6/Δswe1::kanMX6 | This study |
| JPY225 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 ARG4/c HIS4/a ADE2/b Δdmc1::ARG4/Δdmc1::ARG4 Δsum1::kanMX6/Δsum1::kanMX6 | This study |
| JPY65 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 ARG4/c HIS4/a ADE2/b Δdmc1::ARG4/Δdmc1::ARG4 Δswe1::kanMX6/Δswe1::kanMX6 | This study |
| JPY173 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 ARG4/c HIS4/a ADE2/b Δdmc1::ARG4/Δdmc1::ARG4 Δswel::kanMX6/Δswel::kanMX6 Δndt80::LEU2/Δndt80::LEU2 | This study |
| JPY232 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2/leu2 ARG4/c HIS4/a ADE2/b Δdmc1::ARG4/Δdmc1::ARG4 Δsum1::kanMX6/Δsum1::kanMX6 Δndt80::LEU2/Δndt80::LEU2 | This study |
| DKB1170 | MATa/MATα ho::LYS2/ho::LYS2 lys2/lys2 ura3/ura3 leu2::hisG/leu2::hisG arg4-BglII/arg4-NspI his4B::LEU2/his4X::LEU2-URA3 Δrad17::hisG/Δrad17::hisG Δdmc1::ARG4/Δdmc1::ARG4 | D. Bishop (32) |
This strain may carry his4X-ADE2-his4B.
This strain may carry ade2.
This strain may carry arg4-NspI.
Yeast transformations were performed by the lithium acetate method (13). Standard genetic methods were used for mating, sporulation, and random spore disruption.
Media and growth conditions.
SD medium is a minimal medium (2% glucose, 0.7% yeast nitrogen base without amino acids) supplemented with 40 μg of adenine sulfate per ml, 20 μg of l-arginine per ml, 20 μg of l-histidine per ml, 60 μg of l-leucine per ml, 30 μg of l-lysine (mono-HCl) per ml, 20 μg of l-methionine per ml, 50 μg of l-phenylalanine per ml, 200 μg of l-threonine per ml, 40 μg of l-tryptophan per ml, 30 μg of l-tyrosine per ml, and 20 μg of uracil per ml. SD-X medium refers to SD medium that lacks supplement X. Rich medium (yeast extract-peptone-dextrose [YEPD]), presporulation medium (YEPA), and sporulation medium (SPO) were as described previously for SK1-derived strains (18). All yeast cultures were grown at 30°C.
Sporulation of SK1-derived strains was performed as follows. Cells that did not contain a plasmid were taken from a YEPA plate and were grown to late log phase in YEPD, whereas cells containing either the pNDT80-426 or pRS426 plasmid were taken from a YEPA plate and grown in SD-uracil. These cultures were used to inoculate YEPA medium (1:100 dilution). When the YEPA culture reached a density of 1.0 × 107 to 2.0 × 107 cells per ml, the cells were harvested by centrifugation, washed once in 1% potassium acetate, and resuspended in SPO medium at a density of 2.0 × 107 cells per ml. The time of transfer of cells to sporulation medium is referred to as 0 h. The efficiency of ascus formation was assessed by examination of 200 or more cells by light microscopy after 24 h or more in SPO medium.
Plasmids.
pNDT80-426 was constructed by moving a 2.7-kb NotI-SalI fragment from p1-1 (35) into pRS426 cut with NotI and SalI.
RNA isolation and Northern analysis.
RNA preparation from yeast and Northern blot analysis were performed as described previously (18). Gene-specific probes were prepared with templates as follows: NDT80, a 1.2-kb Eco47III-BamHI fragment from pNKY1212 (52); CLB1, a 500-bp EcoRV-EcoRV fragment from pMT417 (provided by M. Tyers); SMK1, an 800-bp StyI-StyI fragment from pLAKK40 (22); SPS1, a 550-bp ClaI-EcoRV fragment from pSPS1-URA3 (12); SPS4, a 521-bp MluI-ClaI fragment from p4LE159 (17); SPS100, a 750-bp BamHI-NcoI fragment from pE18-B8a (25); pC4, an uncharacterized control gene (25).
Microscopy.
For monitoring meiotic divisions, cells were fixed and stained with 4′,6′-diamidino-2-phenylindole (DAPI) as described previously (18) and examined with a Zeiss Axioskop microscope with fluorescence optics. To assess spore wall formation, cells from strains DKB98, DKB608, DKB1170, JPY65, JPY225, and JPY173 were harvested 24 h after transfer to sporulation medium, washed in water, and prepared for electron microscopy. Briefly, cells were fixed for 2 h at room temperature and then overnight at 4°C in a solution containing 3% acrolein, 3% glutaraldehyde, 0.1 M cacodylate, and 1 mM calcium chloride. Cells were washed and postfixed in 1.5% potassium permanganate, 0.1 M cacodylate, and 1 mM calcium chloride for 1 h at 4°C. Cells were then washed again and fixed in 1% osmium tetroxide, 0.1 M cacodylate, 1 mM calcium chloride in the dark for 1 h at room temperature. Samples were then dehydrated through a graded series of ethanol and propylene oxide solutions and embedded in Spurr resin. Thin sections were stained with saturated uranyl acetate and Reynold's lead citrate and examined on a Hitachi H7000 electron microscope.
RESULTS
Overexpression of NDT80 in dmc1/dmc1 cells restores expression of middle sporulation-specific genes and promotes entry into the meiotic division.
Ndt80 is a transcriptional activator that is required for the expression of middle sporulation-specific genes, including the CLB genes. Activation of the meiotic recombination checkpoint leads to reduced transcription of the NDT80 gene, and the Ndt80 that is synthesized is inactive and unable to promote expression of middle sporulation-specific genes (7, 18, 49). We tested to what extent overexpressing NDT80 might suppress checkpoint-mediated arrest. For our phenotypic comparisons, we used a wild-type SK1 diploid strain and SK1-derived dmc1/dmc1 and dmc1/dmc1 rad17/rad17 strains. Mutation of DMC1 leads to the accumulation of unrepaired double-strand breaks, which activates the meiotic recombination checkpoint (4). Mutation of RAD17 prevents transmission of the arrest signal, partially alleviating dmc1-induced pachytene arrest (32).
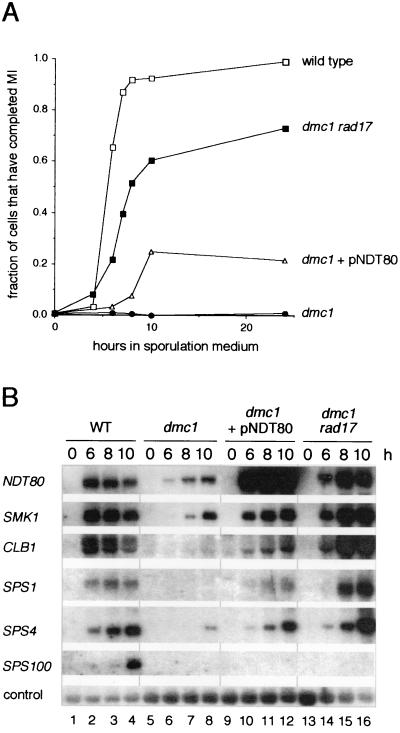
We followed the progress of cells through the meiotic divisions by monitoring separation of chromosomes by fluorescence microscopic examination of cells that had been treated with the DNA-binding dye DAPI. We also monitored spore formation by phase-contrast microscopy to determine if those cells that had completed the meiotic divisions were capable of progressing further in the sporulation program. Ninety percent of wild-type cells underwent the meiotic divisions ≈4 to 8 h after transfer to sporulation medium (Fig. 1A) and had formed mature asci by 24 h (data not shown). Less than 1% of dmc1/dmc1 cells entered the meiotic divisions (Fig. 1A), and neither mature nor immature spores were observed by phase-contrast microscopy (data not shown). In contrast, approximately 20% of dmc1/dmc1 cells that harbored a high- copy plasmid containing the NDT80 gene, under the control of its own sporulation-specific promoter, entered the meiotic divisions, albeit with a delay of ≈4 h relative to wild-type cells (Fig. 1A). None of these cells formed spores as assessed by phase-contrast microscopy (data not shown). By comparison, mutation of RAD17 led to a more effective bypass of the arrest imposed by mutation of DMC1. Seventy percent of dmc1/dmc1 rad17/rad17 cells entered the meiotic divisions and did so with only a 2-h delay relative to wild-type cells (Fig. 1A). Some of these cells formed asci containing immature spores that lacked the clear definition characteristic of mature spores, as assessed by phase-contrast microscopy (data not shown).
FIG. 1.
Cells of a dmc1/dmc1 rad17/rad17 strain and cells of a dmc1/dmc1 strain overexpressing NDT80 enter the meiotic divisions and express middle sporulation-specific genes. (A) Comparison of the efficiency of entry into the meiotic divisions by wild-type cells, dmc1/dmc1 rad17/rad17 cells, dmc1/dmc1 cells containing the high-copy plasmid pNDT80-426 (see Materials and Methods), and dmc1/dmc1 cells containing the vector pRS426. Cells that had been incubated in sporulation medium for the indicated times were fixed, stained with DAPI, and examined by fluorescence microscopy. Cells that appeared binucleate, trinucleate, or tetranucleate were scored as having completed meiosis I (MI). The data presented are representative of at least two trials per strain. (B) Sporulation-specific gene expression in wild-type cells (lanes 1 to 4), dmc1/dmc1 cells containing the pRS426 vector (lanes 5 to 8), dmc1/dmc1 cells containing the high-copy pNDT80-426 plasmid (lanes 9 to 12), and dmc1/dmc1 rad17/rad17 cells (lanes 13 to 16). This Northern filter contained RNA extracted from the indicated strains during vegetative growth (0 h), or 6, 8, or 10 h after transfer to sporulation medium, as noted above the top panel. The filter was hybridized sequentially with radioactively labeled probes specific for NDT80, SMK1, CLB1, SPS1, SPS4, SPS100, and the loading control, pC4 (see Materials and Methods).
We next compared the pattern of premiddle and middle sporulation-specific gene expression by Northern blot analysis of RNA from cells harvested during vegetative growth and at 6, 8, and 10 h after transfer to sporulation medium. This is the period of maximal expression of middle sporulation-specific genes in SK1 strains (18, 35). As observed previously, transcript accumulation from the premiddle genes, NDT80 and SMK1, was reduced to a low level in dmc1-arrested cells, and the middle genes, CLB1, SPS1, and SPS4, and the late gene, SPS100, were not expressed to any significant extent (Fig. 1B, lanes 5 to 8) (7, 18). Expression of premiddle genes and middle genes was at wild-type levels, if not higher, in cells of the dmc1/dmc1 rad17/rad17 strain; maximal expression, however was delayed by approximately 2 h relative to wild-type cells (Fig. 1B, lanes 13 to 16) (18). Although overexpression of NDT80 in dmc1/dmc1 cells led to increased SMK1 expression and promoted expression of CLB1, SPS1, and SPS4, the level of expression was less than in dmc1/dmc1 rad17/rad17 cells (Fig. 1B, lanes 9 to 12). Thus, the presumptive titration of the checkpoint signal by the overexpression of NDT80 in dmc1/dmc1 cells is not as effective in bypassing arrest as blocking the transmission of the checkpoint signal itself, as in dmc1/dmc1 rad17/rad17 cells.
In summary, overexpression of NDT80 in dmc1/dmc1 cells allowed at least a portion of Ndt80 to escape checkpoint-mediated inactivation and promote a low level of expression of at least some middle sporulation-specific genes. We suggest that expression of the CLB genes allowed sufficient Cdc28 activity in some cells to drive them into the meiotic divisions, but only after a significant delay. We presume that the lack of spore formation reflected, at least in part, that expression of middle sporulation-specific genes was not restored to a sufficiently high level.
dmc1/dmc1 sum1/sum1 cells express middle sporulation-specific genes, proceed through the meiotic divisions, and form spores.
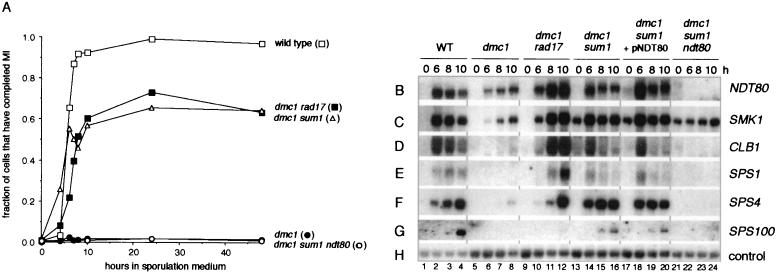
SUM1 encodes a transcriptional regulator that represses expression of some sporulation-specific genes in mitotic cells and prevents premature expression of the NDT80 gene in sporulating cells (35, 51). Lindgren and coworkers (29) discovered that mutation of SUM1 allows dmc1/dmc1 cells to bypass the pachytene arrest signal and to enter the meiotic divisions slightly in advance of wild-type cells but with reduced efficiency and in the absence of expression of middle sporulation-specific genes. In our examination of the phenotype of a dmc1/dmc1 sum1/sum1 strain, we also found that the mutant cells entered the meiotic divisions in advance of wild-type cells; at 4 h after transfer into sporulation medium, 3 and 26% of wild-type and dmc1/dmc1 sum1/sum1 cells, respectively, had entered the meiotic divisions. In contrast to the study of Lindgren and coworkers (29), we found that dmc1/dmc1 sum1/sum1 cells entered the meiotic divisions relatively efficiently, with 22 to 60% of the cells having entered the meiotic divisions at 10 h (Fig. 2A and data not shown). More significantly, we found the premiddle genes, NDT80 and SMK1, and the middle genes, CLB1, SPS1, and SPS4, were expressed in dmc1/dmc1 sum1/sum1 cells at near-wild-type levels (Fig. 2B to F, lanes 13 to 16). Transcript accumulation from SPS1 and SPS4 appeared to reach maximal levels earlier than in both wild-type and dmc1/dmc1 rad17/rad17 cells (Fig. 2E and F and data not shown), and SPS100, a late gene, was also expressed (Fig. 2G, lanes 13 to 16). As observed previously (51), mutation of SUM1 led to derepression of SMK1 in mitotic cells (Fig. 2C, lanes 13, 17, and 21). As would be expected from the already high level of NDT80-dependent gene expression in dmc1/dmc1 sum1/sum1 cells, introduction of a high-copy plasmid containing the NDT80 gene into these cells did not affect the pattern of gene expression (Fig. 2B to G, lanes 17 to 20).
FIG. 2.
Deletion of SUM1 in dmc1/dmc1 cells leads to efficient entry into the meiotic divisions and a high level of NDT80-dependent expression of premiddle and middle sporulation-specific genes. (A) Comparison of the efficiency of entry into the meiotic divisions in wild-type cells, dmc1/dmc1 cells, dmc1/dmc1 sum1/sum1 cells, and dmc1/dmc1 sum1/sum1 ndt80/ndt80 cells. Cells were taken during growth (0 h) or at the indicated times after transfer to sporulation medium, fixed, stained with DAPI, and examined by fluorescence microscopy. Completion of the meiosis I (MI) division was assessed as described in the legend for Fig. 1A. The data for wild-type cells and dmc1/dmc1 rad17/rad17 cells are the same as those presented in Fig. 1A. All data presented are representative of at least two trials per strain. (B to H) Sporulation-specific gene expression in wild-type cells (lanes 1 to 4), dmc1/dmc1 cells (lanes 5 to 8), dmc1/dmc1 rad17/rad17 cells (lanes 9 to 12), dmc1/dmc1 sum1/sum1 cells containing the pRS426 vector (lanes 13 to 16), dmc1/dmc1 sum1/sum1 cells containing the high-copy pNDT80-426 plasmid (lanes 17 to 20), and dmc1/dmc1 sum1/sum1 ndt80/ndt80 cells (lanes 21 to 24). A Northern filter was prepared with RNA purified from these cells harvested during vegetative growth (0 h) or at 6, 8, or 10 h after transfer to sporulation medium, as noted above the top panel (see Materials and Methods). The RNA used in the wild-type and dmc1/dmc1 rad17/rad17 lanes was from the same preparations as those used for the experiment shown in Fig. 1B. The filter was hybridized sequentially with radioactively labeled probes specific for NDT80 (B), SMK1 (C), CLB1 (D), SPS1 (E), SPS4 (F), SPS100 (G), and the loading control, pC4 (H) (see Materials and Methods).
We next constructed a dmc1/dmc1 sum1/sum1 ndt80/ndt80 strain to test whether the ability of dmc1/dmc1 sum1/sum1 cells to bypass the arrest signal required NDT80. Although normally NDT80 is required for entry into pachytene, Lindgren et al. (29) suggested that bypass of dmc1-induced arrest by mutation of SUM1 might allow entry into the meiotic divisions in the absence of Ndt80-promoted middle gene expression. We found, however, that homozygous diploid dmc1 sum1 ndt80 cells were completely defective in entry into the meiotic divisions (Fig. 2A) and did not express CLB1, SPS1, or SPS4 (Fig. 2D to F, lanes 21 to 24). This result, along with our observation that mutation of SUM1 allowed premature expression of NDT80 in sporulating cells (35), suggests that the ability of dmc1/dmc1 sum1/sum1 cells to bypass the arrest signal depends, at least in part, on the presence of Ndt80 prior to establishment of the dmc1-mediated arrest signal. This might allow sufficient Ndt80-dependent CLB gene expression to occur to efficiently drive dmc1/dmc1 sum1/sum1 cells into the meiotic divisions.
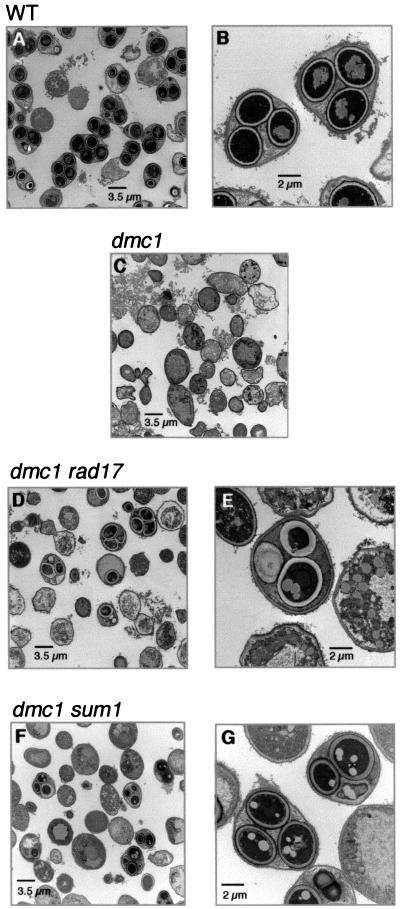
We then compared the extent of spore morphogenesis in dmc1/dmc1 sum1/sum1 cells to that in wild-type, dmc1/dmc1, and dmc1/dmc1 rad17/rad17 cells. In order to more specifically ascertain the extent of spore morphogenesis, we monitored spore wall formation by electron microscopy. Fifty-six percent of wild-type cells that had been in sporulation medium for 24 h formed asci that contained mature spores only; another ≈20% of the cells contained one or more spores that had not yet formed the multilayered spore wall that is characteristic of mature spores (Fig. 3A and B). As expected, cells of the dmc1/dmc1 strain did not form spores, and in only 1% of these cells did we observe any compartmentalization (Fig. 3C). Although dmc1/dmc1 rad17/rad17 cells also do not form mature asci (32), approximately 17% of these cells contained immature or aberrant spores (Fig. 3D and E). The inner layers of the spore walls in some of these spores were very diffuse, and the ascus retained a round shape, rather than condensing as in wild-type cells such that the ascal wall is closely juxtaposed to the spore walls. The dmc1/dmc1 sum1/sum1 cells formed asci that ranged in maturity (Fig. 3F); approximately 27% of these asci contained only immature spores or a mixture of immature and mature spores. However, 6% of the asci were indistinguishable from mature wild-type asci (Fig. 3G).
FIG. 3.
Deletion of SUM1 allows dmc1/dmc1 cells to form mature spores. Cells were fixed and prepared for examination by electron microscopy 24 h after transfer to sporulation medium (see Materials and Methods). (A) A representative field of wild-type cells showing mature asci. (B) A higher-magnification micrograph of two asci containing three mature spores within the plane of the section. Due to the tetrahedral arrangement of mature spores, a fourth spore may be above or below the plane of this section. The spores are enclosed within mature spore walls, and the ascal wall is closely juxtaposed to the spores (see text for details). (C) A representative field of dmc1/dmc1 cells; no spores are present. (D) A representative field of dmc1/dmc1 rad17/rad17 cells showing asci containing spores at various stagesof maturity; no mature asci are present. (E) A higher magnification of a dmc1/dmc1 rad17/rad17 ascus containing one relatively mature spore (bottom) and two immature spores (top). The ascal wall has not condensed around these spores. (F) A representative field of dmc1/dmc1 sum1/sum1 cells showing asci at various stages of maturity. (G) A higher magnification of two dmc1/dmc1 sum1/sum1 asci. The ascus on the left is indistinguishable from a mature wild-type ascus (see panel B); the spores have mature spore walls and the ascal wall is closely juxtaposed to the spores. The ascus on the upper right contains three spores at various stages of maturity.
In summary, we found that dmc1/dmc1 cells that lacked Sum1 activity expressed middle sporulation-specific genes, entered the meiotic divisions efficiently, and in some cases formed asci containing mature spores. Both middle gene expression and exit from pachytene occurred slightly earlier than in wild-type cells and was dependent on NDT80.
dmc1/dmc1 swe1/swe1 cells progress into the meiotic divisions but expression of middle genes is not restored.
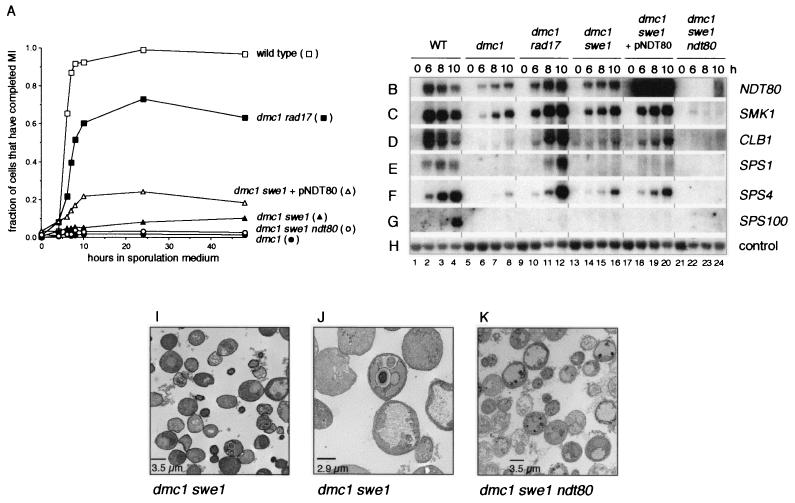
Although SWE1 is not required for sporulation, pachytene arrest in response to the meiotic recombination checkpoint requires that Swe1 phosphorylate Cdc28 at Y19 and thereby inhibit its activity (5, 27). We found that mutation of SWE1 did not bypass the dmc1-mediated pachytene arrest in SK1-derived strains as efficiently as reported for YAB36-derived strains (27). Whereas Leu and Roeder (27) found that YAB36-derived dmc1/dmc1 swe1/swe1 cells enter the meiotic divisions almost as efficiently as wild-type cells, only 10 to 30% of SK1-derived dmc1/dmc1 swe1/swe1 cells entered the meiotic divisions (Fig. 4A and data not shown). In the YAB36-derived strain, however, dmc1-mediated arrest is not complete; more than 10% of YAB36-derived dmc1/dmc1 cells enter the meiotic divisions, whereas less than 1% of SK1-derived dmc1/dmc1 cells do so (Fig. 4A) (27). dmc1/dmc1 swe1/swe1 cells of both strains showed a long delay in entry into the meiotic divisions (Fig. 4A) (27).
FIG. 4.
Examination of the effect of deletion of SWE1 on dmc1-induced arrest. (A) Comparison of the efficiency of entry into the meiotic divisions in wild-type cells, dmc1/dmc1 cells, dmc1/dmc1 swe1/swe1 cells containing the pRS426 vector, dmc1/dmc1 swe1/swe1 cells containing the high-copy pNDT80-426 plasmid, and dmc1/dmc1 swe1/swe1 ndt80/ndt80 cells. Cells were taken during growth (0 h) or at the indicated times after transfer to sporulation medium, fixed, stained with DAPI, and examined by fluorescence microscopy. Completion of the meiosis I (MI) division was assessed as described in the legend for Fig. 1A. The data for wild-type cells, dmc1/dmc1 cells, and dmc1/dmc1 rad17/rad17 cells are the same as those presented in Fig. 2A. All data presented are representative of at least two trials per strain. (B to H) Deletion of SWE1 does not restore a wild-type level of middle gene expression to dmc1/dmc1 cells. A Northern filter was prepared with RNA purified from wild-type cells (lanes 1 to 4), dmc1/dmc1 cells (lanes 5 to 8), dmc1/dmc1 rad17/rad17 cells (lanes 9 to 12), dmc1/dmc1 swe1/swe1 cells containing the vector plasmid pRS426 (lanes 13 to 16) or the high-copy plasmid pNDT80-426 (lanes 17 to 20), and dmc1/dmc1 swe1/swe1 ndt80/ndt80 cells (lanes 21 to 24) harvested during vegetative growth (0 h) or at 6, 8, or 10 h after transfer to sporulation medium, as noted above the top panel (see Materials and Methods). The RNA used in the wild-type, dmc1/dmc1, and dmc1/dmc1 rad17/rad17 lanes was from the same preparations as those used for the experiment shown in Fig. 2B to H. The filter was hybridized sequentially with radioactively labeled probes specific for NDT80 (B), SMK1 (C), CLB1 (D), SPS1 (E), SPS4 (F), SPS100 (G), and the loading control, pC4 (H) (see Materials and Methods). (I to K) Initiation of spore morphogenesis in dmc1/dmc1 swe1/swe1 cells is dependent on NDT80. Cells of a dmc1/dmc1 swe1/swe1 strain (I and J) and of a dmc1/dmc1 swe1/swe1 ndt80/ndt80 strain (K) were fixed and prepared for examination by electron microscopy 24 h after transfer to sporulation medium (see Materials and Methods). (I) A representative field of dmc1/dmc1 swe1/swe1 cells shows no mature asci; some cells contain prospore-like compartments. (J) A higher-magnification micrograph showing a dmc1/dmc1 swe1/swe1 cell that contains immature and aberrant spore-like compartments. (K) A representative field of dmc1/dmc1 swe1/swe1 ndt80/ndt80 cells shows no asci or any cells that have initiated spore morphogenesis.
Expression of NDT80, SMK1, CLB1, SPS1, and SPS4 in swe1/swe1 cells in sporulation medium was similar to that in wild-type cells (data not shown), whereas expression of these genes in dmc1/dmc1 swe1/swe1 cells was significantly reduced below wild-type levels and maximal accumulation was delayed even further than in dmc1/dmc1 rad17/rad17 cells (Fig. 4B to F, lanes 13 to 16). Spore formation was also impaired; as assessed by electron microscopy, only 5% of dmc1/dmc1 swe1/swe1 cells showed some compartmentalization, most of which appeared aberrant, and no mature spores were seen (Fig. 4I and J).
Role of NDT80 in promoting entry into the meiotic divisions in dmc1/dmc1 swe1/swe1 cells.
We next tested whether Ndt80-dependent regulation of CLB gene expression was required for the bypass of pachytene arrest in dmc1/dmc1 swe1/swe1 cells. Mutation of NDT80 almost completely abolished the ability of dmc1/dmc1 swe1/swe1 cells to enter the meiotic divisions (Fig. 4A). However, we consistently found that 2 to 4% of cells of the dmc1/dmc1 swe1/swe1 ndt80/ndt80 strain entered the meiotic divisions, whereas only 1 to 2% of dmc1/dmc1 cells did so (Fig. 4A). We note that in cells of the latter strain, but not the former, the nuclei appeared to become fragmented late during sporulation. This may have led to an overestimate in the number of dmc1/dmc1 cells that had segregated their chromosomes. There was no middle gene expression, however, in the triple mutant cells (Fig. 4B to F, lanes 21 to 24), and the very immature spores that were seen occasionally in dmc1/dmc1 swe1/swe1 cells (Fig. 4I and J) were never seen in dmc1/dmc1 swe1/swe1 ndt80/ndt80 cells (Fig. 4K).
Leu and Roeder (27) have shown that overexpression of CLB1 in a dmc1/dmc1 swe1/swe1 strain restores wild-type kinetics of meiotic division. Because Ndt80 is responsible for CLB1 upregulation during sporulation, we tested whether overexpression of NDT80 would have the same effect. We found that dmc1/dmc1 swe1/swe1 cells that contained NDT80 on a high-copy plasmid entered the meiotic divisions with improved kinetics and efficiency relative to dmc1/dmc1 swe1/swe1 cells that did not overexpress NDT80 (Fig. 4A). However, there was only a very modest increase in expression of CLB1 and other middle genes (Fig. 4B to F, lanes 17 to 20) relative to dmc1/dmc1 swe1/swe1 cells (Fig. 4B to F, lanes 13 to 16), and only 1% of these cells formed mature asci as assessed by phase-contrast microscopy (data not shown).
DISCUSSION
A model for the role of Ndt80, Sum1, and Swe1 as targets of the meiotic recombination checkpoint.
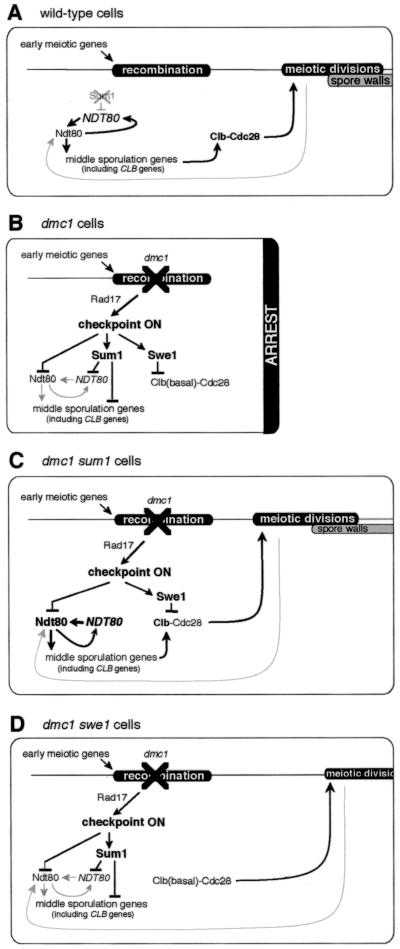
In this study we have investigated the extent to which the meiotic recombination checkpoint can be bypassed by manipulation of three of its targets, NDT80, SUM1, and SWE1. Based on our results and the results of other studies (27, 29, 49), we describe below a model of the regulatory events that control entry into the meiotic divisions and subsequent spore formation (Fig. 5 ). This model takes into consideration the idea that Ndt80 and Sum1 act as competing regulators of at least some MSE-regulated sporulation-specific genes (29). The expression level of each such gene is dictated, at least in part, by the relative affinity of Ndt80 and Sum1 for its MSE site and by the amount of Ndt80 and of Sum1 in the cells (51). We propose that early during sporulation, at the time that cells begin to engage in meiotic recombination, Sum1 maintains repression of NDT80 and some middle sporulation-specific genes (35, 51). At about the time that wild-type cells begin processing recombination intermediates, Sum1 is destabilized (Fig. 5A) (29). This allows expression of NDT80 (35), which upregulates its own expression. As the level of Ndt80 increases and the level of Sum1 declines, a subset of MSE-regulated sporulation-specific genes, including CLB1, CLB3, CLB4, CLB5, and CLB6, is expressed. Accumulation of Clb1, Clb3, and Clb4 provides the Clb-Cdc28 activity that is required for cells to enter the meiotic divisions (10, 14). We suggest that exit from pachytene provides a signal that further upregulates the expression of NDT80. This feedback mechanism explains our observation that dmc1/dmc1 swe1/swe1 cells are able to express middle genes in an Ndt80-dependent fashion, albeit at a low level. It is also possible that exit from pachytene provides a signal that attenuates the ability of cells to respond to the meiotic recombination checkpoint signal (see below). However, the efficient and timely expression of middle sporulation-specific genes is required for the formation of mature asci.
FIG. 5.
Model for the roles of NDT80, SUM1, and SWE1 as targets of the meiotic recombination checkpoint. See text for details. The top horizontal line denotes the timing of landmark events during sporulation. Lines terminating in arrowheads indicate that the expression of a gene or the function of a protein has been activated, whereas lines terminating in bars indicate that the expression of a gene or the function of a protein has been inhibited. Early during sporulation in wild-type cells, Sum1 represses expression of NDT80 and middle genes. (A) Towards the end of prophase in wild-type cells, Sum1 is destabilized, which allows expression of NDT80. Ndt80 then activates expression of middle sporulation-specific genes, including CLB genes. This leads to an increase in Clb-Cdc28 activity, which drives cells out of pachytene and into the meiotic divisions. The gray arrow refers to aputative feedback mechanism that upregulates Ndt80 in cells that have exited pachytene. (B) In dmc1/dmc1 cells, activation of the checkpoint results in inactivation of Ndt80 and stabilization of Sum1, which prevent expression of middle sporulation-specific genes, including CLB genes. Concomitant stabilization of Swe1 ensures that Clb-Cdc28 is inactive and that cells arrest at pachytene. (C) The absence of Sum1 in dmc1/dmc1 sum1/sum1 cells allows expression of NDT80 prior to activation of the checkpoint signal. Additionally, sufficient Ndt80 accumulates such that the ability of the checkpoint to inhibit the activity of Ndt80 is at least in part titrated. Middle sporulation-specific genes, including CLB genes, are expressed. Increased Clb levels promote entry into the meiotic divisions despite the presence of active Swe1; the high level of expression of middle sporulation-specific genes promotes spore morphogenesis. (D) In dmc1/dmc1 swe1/swe1 cells, inhibition of Clb-Cdc28 is relieved, but inactivation of Ndt80 and stabilization of Sum1 are initially maintained. In some cells there is sufficient Clb-Cdc28 activity to promote exit from pachytene. We speculate that these cells do not progress into the meiotic divisions until a putative feedback mechanism attenuates the checkpoint signal, allowing increased Ndt80 activity. The subsequent increase in the level of Clb's drives the cells into the meiotic divisions.
The meiotic recombination checkpoint is activated by the persistence of recombination intermediates or defects in synapsis of chromosomes and mediates at least three responses that ultimately result in the arrest of the sporulation program at the pachytene stage. First, Sum1 is stabilized (29) which, in turn, results in the continued repression of NDT80 (Fig. 5B). Second, any Ndt80 that has been synthesized is inactivated (7, 18, 49). Third, Swe1 kinase accumulates in a hyperphosphorylated form and inactivates Cdc28 kinase by phosphorylation (27). As described below, we propose that manipulations that upregulate Clb-Cdc28 activity in cells in which the meiotic recombination checkpoint has been activated allow these cells to enter the meiotic divisions but do not permit subsequent spore formation. We suggest that delayed and/or reduced expression of middle sporulation-specific genes prevents spore formation from proceeding in these cells. In contrast, genetic manipulations that increase Ndt80-dependent middle gene expression earlier during sporulation in checkpoint-activated cells promote the formation of spores.
Effect of mutation of SUM1.
In our studies, we found that the most effective bypass of dmc1-induced pachytene arrest occurred on mutation of SUM1. Because mutation of SUM1 allows premature expression of NDT80 in sporulating cells (35), we suggest that active Ndt80 accumulates prior to establishment of the dmc1-mediated arrest signal in dmc1/dmc1 sum1/sum1 cells. Indeed, we have also found that expression of NDT80 from the promoter of the early meiotic gene, HOP1, allows dmc1/dmc1 cells to express the middle sporulation-specific gene SPS4 (J. Pak, unpublished data). Additionally, in dmc1/dmc1 sum1/sum1 cells, Ndt80 no longer has to compete with Sum1 for occupancy of MSE sites. We propose that this allows sufficient Ndt80-dependent CLB gene expression to drive dmc1/dmc1 sum1/sum1 cells into the meiotic divisions (Fig. 5C). We also suggest that the premature expression of the CLB genes in dmc1/dmc1 sum1/sum1 cells is responsible for the precocious entry of these cells into the meiotic divisions and that the timely expression of other middle sporulation-specific genes allows some of these cells to form asci containing mature spores.
We have observed that the complete absence of Sum1, a target of the meiotic recombination checkpoint, in dmc1/dmc1 sum1/sum1 cells provides a more effective bypass of the checkpoint than does inactivation of the checkpoint itself in dmc1/dmc1 rad17/rad17 cells. Although dmc1/dmc1 rad17/rad17 cells undergo meiosis efficiently, entry into the meiotic divisions is delayed and mature spores do not form (references 15, 29, and 32 and this study). As suggested above, this difference could reflect the premature expression of NDT80 in dmc1/dmc1 sum1/sum1 cells. We hypothesize that there exists a window of time during which spore morphogenesis genes must be expressed for mature asci to form and that the absence of Sum1 allows expression of these genes in a timely manner. In contrast, delayed expression of these genes in dmc1/dmc1 rad17/rad17 cells disrupts the coordination of spore morphogenesis events. Other possibilities can be considered. For example, RAD17, in addition to its checkpoint function, has a role in synapsis (15); it is possible that defects in this second function affect spore formation in dmc1/dmc1 rad17/rad17 cells in a manner that is distinct from the Rad17-dependent checkpoint pathway. Alternatively, dmc1/dmc1 cells may transmit a checkpoint signal to both a Rad17-dependent pathway and a putative Rad17-independent pathway, both of which regulate Sum1. The observation by Lindgren et al. (29) that RAD17 is required for the transient destabilization of Sum1 in sporulating cells is consistent with the existence of a Rad17-dependent pathway. Our observation that deletion of SUM1 bypasses pachytene arrest more efficiently than mutation of RAD17 suggests the existence of both a Rad17-dependent and a Rad17-independent checkpoint pathway.
In contrast to our results, Lindgren et al. (29) found that cells of their SK1-derived dmc1/dmc1 sum1/sum1 strain (EWY90) do not express middle sporulation-specific genes, are less efficient than dmc1/dmc1 rad17/rad17 cells in completing the meiotic divisions, and fail to form any wild-type asci. We suggest that subtle differences in laboratory procedures account for these different behaviors, as we have found that cells of the EWY90 strain express middle sporulation-specific genes (data not shown). Based on their results, Lindgren et al. (29) suggested that dmc1/dmc1 sum1/sum1 cells might enter the meiotic divisions in an NDT80-independent manner. We have found that this is not the case; cells of a dmc1/dmc1 sum1/sum1 ndt80/ndt80 strain failed to enter the meiotic divisions.
Effect of overexpression of NDT80.
Previous studies have indicated that activation of the meiotic recombination checkpoint leads to reduced expression of NDT80 and to inactivation of Ndt80 (7, 18, 49). Tung et al. (49) have shown that phosphorylation of Ndt80 is prevented by the meiotic recombination checkpoint and that the checkpoint can be bypassed by overexpressing NDT80. This suggests that the mechanism that inhibits Ndt80 activity is limiting and can be titrated in the presence of excess Ndt80 (49). Our results are consistent with this interpretation; overexpression of NDT80 in dmc1/dmc1 cells led to an increase in middle gene expression and entry into the meiotic divisions but at efficiencies that were lower than those observed in wild-type or dmc1/dmc1 rad17/rad17 cells, suggesting that Ndt80 is, indeed, posttranslationally inactivated in dmc1/dmc1 cells but not in dmc1/dmc1 rad17/rad17 cells. However, because Sum1 and Ndt80 compete for binding to MSE sites and act in opposition to repress and activate, respectively, the expression of a set of sporulation-specific genes (51), we cannot exclude the possibility that the apparent posttranslational inhibition of Ndt80 is a reflection of stabilization of Sum1 in arrested cells. It seems likely that the Ndt80/Sum1 ratio must reach a certain level before Ndt80 can outcompete Sum1 for binding to MSE sites. In dmc1/dmc1 cells overexpressing NDT80 the checkpoint would maintain the stability of Sum1, whereas in dmc1/dmc1 rad17/rad17 cells the checkpoint signal would no longer be transduced and Sum1 would be destabilized. Future experiments will indicate whether the Ndt80/Sum1 ratio is greater in the latter cells than in the former.
Effect of mutation of SWE1.
Because Clb-Cdc28 activity is not required for middle gene expression (18), we did not expect that mutation of SWE1 and loss of inhibitory phosphorylation of Cdc28 would restore middle gene expression in dmc1/dmc1 cells. We found, however, that premiddle and middle gene transcript levels were modestly higher in dmc1/dmc1 swe1/swe1 cells than in dmc1/dmc1 cells. Approximately 10% of dmc1/dmc1 swe1/swe1 cells entered the meiotic divisions, although these cells did not form mature asci. We suggest that a low fraction of dmc1/dmc1 swe1/swe1 cells are capable of exiting pachytene due to the low basal level of Clb's in these cells and the release of Clb-Cdc28 from Swe1-mediated inhibition (27). We propose that once these cells have exited pachytene, a feedback loop acts to increase the activity of Ndt80 and additionally, or alternatively, attenuates the response to the checkpoint signal, or the checkpoint signal itself. Active Ndt80 then upregulates expression of middle genes, including the CLBs (Fig. 5D). The resultant increase in Clb levels drives the cells into the meiotic divisions. We assume, however, that either the reduction or the delay in the expression of other middle genes in dmc1/dmc1 swe1/swe1 cells results in the inability of these cells to form mature asci. Previous studies have also led to the conclusion that accumulation of active Cdc28 may act in a feedback pathway to upregulate Ndt80 activity (27, 49). This feedback pathway most likely does not entail the direct phosphorylation of Ndt80 by Cdc28; phosphorylated forms of Ndt80 have been shown to persist in cells that do not contain active Cdc28 (49). As would be expected based on this model, the presence of NDT80 on a high-copy-number plasmid significantly reduced the delay in the onset of the meiotic divisions in dmc1/dmc1 swe1/swe1 cells. This is presumably due to Ndt80-mediated CLB gene expression and is similar to the observations that high-copy CLB1 improves the efficiency and advances the kinetics of entry in the meiotic divisions in dmc1/dmc1 swe1/swe1 cells (27) and that high-copy NDT80 improved the kinetics of entry of zip1/zip1 cells into the meiotic divisions (49).
Activation of Swe1 by the meiotic recombination checkpoint is not sufficient to arrest cells at pachytene in dmc1/dmc1 sum1/sum1 cells or in dmc1/dmc1 cells overexpressing NDT80. This indicates that the inappropriate expression of the CLB genes in these dmc1/dmc1 cells generates more Clb-Cdc28 activity than can be inhibited by Swe1. As mentioned above, the initial activity of Clb-Cdc28 in these cells might be augmented as the cells exit pachytene due to a putative attenuation of the checkpoint signal. It is also possible that Clb-Cdc28 activity might act in a feedback loop and increase its own activity by inhibiting Swe1.
We note that although mutation of NDT80 abolished middle gene expression in dmc1/dmc1 swe1/swe1 cells and severely reduced the number of cells that entered the meiotic divisions, ≈3 to 4% of dmc1/dmc1 swe1/swe1 ndt80/ndt80 cells nonetheless entered the meiotic divisions, whereas less than 2% of dmc1/dmc1 cells entered the meiotic divisions. This is consistent with the notion that the uninduced level of Clb's in at least a few cells is sufficient to allow them to progress into the meiotic divisions if Swe1-dependent inhibition of Cdc28 is prevented.
Acknowledgments
We thank Ed Winter and his coworkers for communicating results prior to publication and for helpful discussions. We are most appreciative of the help and advice provided by Steve Doyle of the Microscopy Imaging Laboratory of the Faculty of Medicine, University of Toronto, and we thank John Glover for the use of his fluorescence microscope. We thank Helena Friesen and Ghadeer Shubassi for their helpful comments on the manuscript.
This work was supported by a Canadian Institute of Health Research grant (MOP-6826) to J.S. J.P. was supported in part by an Ontario Government Scholarship for Science and Technology and by a University of Toronto Open Fellowship.
REFERENCES
- 1.Amon, A., U. Surana, I. Muroff, and K. Nasmyth. 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355:368-371. [DOI] [PubMed] [Google Scholar]
- 2.Bailis, J. M., and G. S. Roeder. 2000. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell 101:211-221. [DOI] [PubMed] [Google Scholar]
- 3.Bailis, J. M., A. V. Smith, and G. S. Roeder. 2000. Bypass of a meiotic checkpoint by overproduction of meiotic chromosomal proteins. Mol. Cell. Biol. 20:4838-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, D. K., D. Park, L. Xu, and N. Kleckner. 1992. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69:439-456. [DOI] [PubMed] [Google Scholar]
- 5.Booher, R. N., R. J. Deshzies, and M. W. Kirschner. 1993. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12:3417-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi, M. H., and D. Shore. 1996. SUM1-1, a dominant suppressor of SIR mutations in Saccharomyces cerevisiae, increases transcriptional silencing at telomeres and HM mating-type loci and decreases chromosome stability. Mol. Cell. Biol. 16:4281-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, S., and I. Herskowitz. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell 1:685-696. [DOI] [PubMed] [Google Scholar]
- 8.Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, D. J., and J. F. Giménez-Abián. 2000. Checkpoints controlling mitosis. Bioessays 22:351-363. [DOI] [PubMed] [Google Scholar]
- 10.Dahmann, C., and B. Futcher. 1995. Specialization of B-type cyclins for mitosis or meiosis in S. cerevisiae. Genetics 140:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foiani, M., A. Pellicioli, M. Lopes, C. Lucca, M. Ferrari, G. Liberi, M. Falconi, and P. Plevani. 2000. DNA damage checkpoints and DNA replication controls in Saccharomyces cerevisiae. Mutat. Res. 451:187-196. [DOI] [PubMed] [Google Scholar]
- 12.Friesen, H., R. Lunz, S. Doyle, and J. Segall. 1994. Mutation of the SPS1-encoded protein kinase of Saccharomyces cerevisiae leads to defects in transcription and morphology during spore formation. Genes Dev. 8:2162-2175. [DOI] [PubMed] [Google Scholar]
- 13.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandin, N., and S. I. Reed. 1993. Differential function and expression of Saccharomyces cerevisiae B-type cyclins in mitosis and meiosis. Mol. Cell. Biol. 13:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grushcow, J. M., T. M. Holzen, K. J. Park, T. Weinert, M. Lichten, and D. K. Bishop. 1999. Saccharomyces cerevisiae checkpoint genes MEC1, RAD17 and RAD24 are required for normal meiotic recombination partner choice. Genetics 153:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246:629-634. [DOI] [PubMed] [Google Scholar]
- 17.Hepworth, S. R., L. K. Ebisuzaki, and J. Segall. 1995. A 15-base-pair element activates the SPS4 gene midway through sporulation in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:3934-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hepworth, S. R., H. Friesen, and J. Segall. 1998. NDT80 and the meiotic recombination checkpoint regulate expression of middle sporulation-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:5750-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong, E.-J. E., and G. S. Roeder. 2002. A role for Ddc1 in signaling meiotic double-strand breaks at the pachytene checkpoint. Genes Dev. 16:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klar, A. J., S. N. Kakar, J. M. Ivy, J. B. Hicks, G. P. Livi, and L. M. Miglio. 1985. SUM1, an apparent positive regulator of the cryptic mating-type loci in Saccharomyces cerevisiae. Genetics 111:745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knop, M., and K. Strasser. 2000. Role of the spindle pole body of yeast in mediating assembly of the prospore membrane during meiosis. EMBO J. 19:3657-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisak, L., R. Strich, R. S. Winters, J. P. Hall, M. J. Mallory, D. Kreitzer, R. S. Tuan, and E. Winter. 1994. SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 8:2151-2161. [DOI] [PubMed] [Google Scholar]
- 23.Kupiec, M., B. Byers, R. E. Esposito, and A. P. Mitchell. 1997. Meiosis and sporulation in Saccharomyces cerevisiae, p. 899-1036. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular biology of the yeast Saccharomyces: cell cycle and cell biology. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Laurenson, P., and J. Rine. 1991. SUM1-1: a suppressor of silencing defects in Saccharomyces cerevisiae. Genetics 129:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law, D. T. S., and J. Segall. 1988. The SPS100 gene of Saccharomyces cerevisiae is activated late in the sporulation process and contributes to spore wall maturation. Mol. Cell. Biol. 8:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leu, J.-Y., P. R. Chua, and G. S. Roeder. 1998. The meiosis-specific Hop2 protein of S. cerevisiae ensures synapsis between homologous chromosomes. Cell 94:375-386. [DOI] [PubMed] [Google Scholar]
- 27.Leu, J.-Y., and G. S. Roeder. 1999. The pachytene checkpoint in S. cerevisiae depends on Swe1-mediated phosphorylation of the cyclin-dependent kinase Cdc28. Mol. Cell 4:805-814. [DOI] [PubMed] [Google Scholar]
- 28.Lew, D. J. 2000. Cell-cycle checkpoints that ensure coordination between nuclear and cytoplasmic events in Saccharomyces cerevisiae. Curr. Opin. Genet. Dev. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 29.Lindgren, A., D. Bungard, M. Pierce, J. Xie, A. Vershon, and E. Winter. 2000. The pachytene checkpoint in Saccharomyces cerevisiae requires the Sum1 transcriptional repressor. EMBO J. 19:6489-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livi, G. P., J. B. Hicks, and A. J. Klar. 1990. The SUM1-1 mutation affects silent mating-type gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 32.Lydall, C., Y. Nikolsky, D. K. Bishop, and T. Weinert. 1996. A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature 383:840-843. [DOI] [PubMed] [Google Scholar]
- 33.Murakami, H., and P. Nurse. 2000. DNA replication and damage checkpoints and meiotic cell cycle controls in the fission and budding yeasts. Biochem. J. 349:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neiman, A. M. 1998. Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J. Cell Biol. 140:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pak, J., and J. Segall. 2002. Regulation of the premiddle and middle phases of expression of the NDT80 gene during sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol. 22:6416-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Primig, M., R. M. Williams, E. A. Winzeler, G. G. Tevzadze, A. R. Conway, S. Y. Hwang, R. W. Davis, and R. E. Esposito. 2000. The core meiotic transcriptome in budding yeast. Nat. Genet. 26:415-423. [DOI] [PubMed] [Google Scholar]
- 37.Rockmill, B., M. Sym, H. Scherthan, and G. S. Roeder. 1995. Roles for two RecA homologs in promoting meiotic chromosome synapsis. Genes Dev. 9:2684-2695. [DOI] [PubMed] [Google Scholar]
- 38.Roeder, B. S., and J. M. Bailis. 2000. The pachytene checkpoint. Trends Genet. 16:395-403. [DOI] [PubMed] [Google Scholar]
- 39.San-Segundo, P. A., and G. S. Roeder. 1999. Pch2 links chromatin silencing to meiotic checkpoint control. Cell 97:313-324. [DOI] [PubMed] [Google Scholar]
- 40.San-Segundo, P. A., and G. S. Roeder. 2000. Role for the silencing protein Dot1 in meiotic checkpoint control. Mol. Biol. Cell 11:3601-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuster, E. O., and B. Byers. 1989. Pachytene arrest and other meiotic effects of the start mutations in Saccharomyces cerevisiae. Genetics 123:29-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits, G. J., H. van den Ende, and F. M. Klis. 2001. Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology 147:781-794. [DOI] [PubMed] [Google Scholar]
- 43.Sorger, P. K., and A. W. Murray. 1992. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature 355:365-368. [DOI] [PubMed] [Google Scholar]
- 44.Storlazzi, A., L. Xu, A. Schwacha, and N. Kleckner. 1996. Synaptonemal complex (SC) component Zip1 plays a role in meiotic recombination independent of SC polymerization along the chromosome. Proc. Natl. Acad. Sci. USA 93:9043-9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sym, M., J. Engebrecht, and G. S. Roeder. 1993. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell 72:365-378. [DOI] [PubMed] [Google Scholar]
- 46.Sym, M., and G. S. Roeder. 1994. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell 79:283-292. [DOI] [PubMed] [Google Scholar]
- 47.Tachikawa, H., A. Bloecher, K. Tatchell, and A. M. Neiman. 2001. A Gip1p-Glc7p phosphatase complex regulates septin organization and spore wall formation. J. Cell Biol. 155:797-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung, K.-S., and G. S. Roeder. 1998. Meiotic chromosome morphology and behavior in zip1 mutants of Saccharomyces cerevisiae. Genetics 149:817-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tung, K.-S., E.-J. Hong, and G. S. Roeder. 2000. The pachytene checkpoint prevents accumulation and phosphorylation of the meiosis-specific transcription factor Ndt80. Proc. Nat. Acad. Sci. USA 97:12187-12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woltering, D., B. Baumgartner, S. Bagchi, B. Larking, J. Loidl, T. de los Santos, and N. M. Hollingsworth. 2000. Meiotic segregation, synapsis, and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol. Cell. Biol. 20:6646-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie, J., M. Pierce, V. Gailus-Durner, M. Wagner, E. Winter, and A. K. Vershon. 1999. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 18:6448-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, L., M. Ajimura, R. Padmore, C. Klein, and N. Kleckner. 1995. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:6572-6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, L., B. M. Weiner, and N. Kleckner. 1997. Meiotic cells monitor the status of the interhomolog recombination complex. Genes Dev. 11:106-118. [DOI] [PubMed] [Google Scholar]
- 54.Zickler, D., and N. Kleckner. 1998. The leptotene-zygotene transition of meiosis. Annu. Rev. Genet. 32:619-697. [DOI] [PubMed] [Google Scholar]