Figure 2.
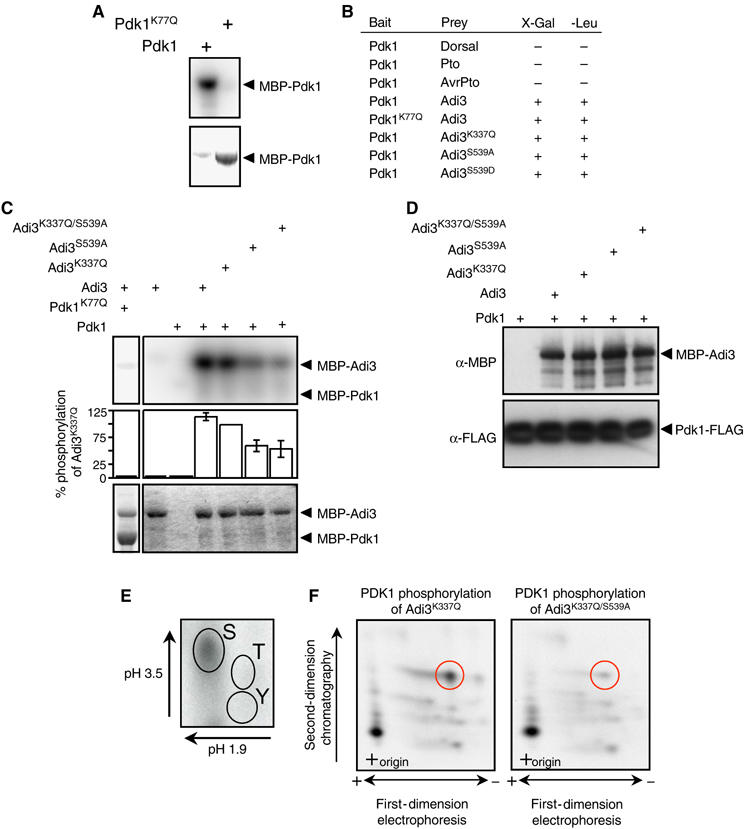
Pdk1 kinase activity and interaction with Adi3. In (A) and (C), the top panel represents the kinase assay (phosphorimage) and the bottom panel the assay input (Coomassie-stained gel). (A) Tomato Pdk1 is a functional protein kinase. Analysis of kinase-active and -deficient MBP-Pdk1 fusion proteins by in vitro autophosphorylation assays is shown. (B) Pdk1 interacts with Adi3 in the Y2H assay. The indicated bait and prey constructs were tested in the Y2H assay for expression of the lacZ gene on X-Gal plates or leucine prototrophy (+, interaction; −, no interaction). (C) Pdk1 phosphorylation of Adi3 in vitro. MBP-Adi3 proteins were used as substrates for kinase-active MBP-Pdk1 protein. (D) In vitro co-immunoprecipitation of Pdk1 and Adi3. MBP-Adi3 and Pdk1-FLAG proteins were co-precipitated using α-FLAG agarose and analyzed by Western blot. (E) Pdk1 phosphorylates Adi3 on Ser residues. Pdk1 phosphorylated Adi3 was analyzed by phosphoamino acid analysis; autoradiograph is shown. (F) Pdk1 phosphorylates Adi3 at S539 on one of two tryptic peptides. Pdk1 phosphorylated Adi3 was digested with trypsin and analyzed by phosphopeptide analysis; autoradiograph is shown.
