Figure 3.
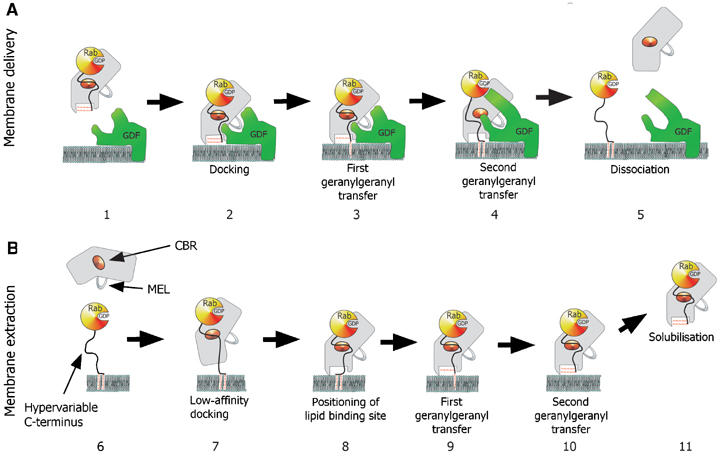
Model for the GDI-mediated Rab/Ypt interaction with the putative Rab receptors and membranes. (A) GDI-mediated delivery of prenylated RabGTPases to the membrane is proposed to involve docking of the Rab:GDI complex with a putative membrane Rab recruitment/GDF via a protein:protein interaction (2). The docked complex undergoes a conformational change, which leads to the transfer of the first and then the second geranylgeranyl moiety into the membrane and subsequently to the release of the Rab C-terminus from the CBR (3 and 4). Finally, GDI is released into the cytosol and the Rab protein enters its functional cycle. (B) GDI-mediated extraction of prenylated RabGTPases from the membrane. Initial recognition is mediated by the low-affinity interaction of the Rab-binding platform with the CBR of GDI (7). This leads to the positioning of GDI domain II on the lipid bilayer over the buried geranylgeranyl moieties of the Rab protein (8). The geranylgeranyl lipids are transferred from the membrane to the lipid binding sites on GDI in two consecutive steps (9 and 10), leading to dissociation of the complex from membrane (11).
