Figure 4.
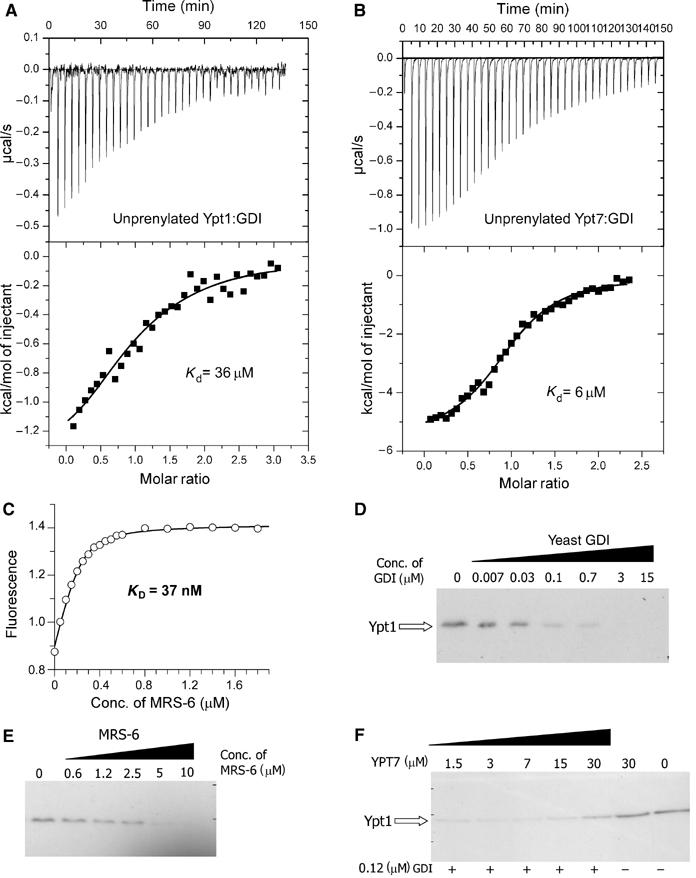
Interaction of GDI and Mrs6 with unprenylated and membrane-bound Ypt proteins. (A) ITC titration of Ypt1 with increasing concentrations of GDI. Fitting of the data led to a Kd value of 36 μM. (B) Same as in (A) but with Ypt7 (Kd=6 μM). (C) A representative fluorescence titration of dans_Ypt1 with Mrs6. The concentration of dans_YPT1 was 240 nM. The fluorescence of the dansyl group was excited at 280 nm via FRET from tryptophanes and the emission was collected at 490 nm. The data were corrected for unspecific fluorescence increase and analysed as described under ‘Materials and methods', leading to a Kd value of 37 nM. (D) Extraction of Ypt1 from S. cerevisiae membranes with yeast GDI. The isolated membrane fraction was incubated with various concentrations of recombinant GDI and the fraction of membrane-associated Ypt1 was determined by Western blotting as described in ‘Materials and methods'. (E) Same as in (D) but the membranes were incubated with recombinant Mrs6. Complete extraction of Ypt1 at high concentrations is not in contradiction to the model calculations, since these were made for specific values of the effective membrane concentration and the affinity of geranylgeranyl residues for membranes, which are not likely to apply here. (F) Unprenylated Ypt7 is able to interfere with the ability of GDI to extract membrane-bound Ypt1. S. cerevisiae membranes were incubated with 120 nM GDI and increasing concentrations of Ypt7. The sample in lane 6 was incubated with 30 μM of Ypt7 and no GDI, while the sample in lane 7 was incubated with buffer alone.
