Abstract
Objective:
The aim of this study was to investigate the prognostic relevance of lymphangiogenesis and lymphovascular invasion in a large cohort of breast cancer patients.
Introduction:
Invasion of tumor cells into blood and lymphatic vessels is one of the critical steps for metastasis. The presence or absence of lymph node metastasis is one of the main decision criteria for further therapy. One shortcoming of previous morphologic studies was the lack of specific markers that could exact discriminate between blood and lymphatic vessels. The aim of this study was to evaluate the prognostic relevance of lymphangiogenesis and lymphovascular invasion in breast cancer patients.
Methods:
We investigated 374 tissue specimens of patients suffering from invasive breast cancer by immunostaining for the lymphatic endothelial specific marker podoplanin. Lymphangiogenesis, quantified by evaluating the lymphatic microvessels density (LMVD), and lymphovascular invasion (LVI) were correlated with various clinical parameters and prognostic relevance.
Results:
LMVD correlated significantly with LVI (P = 0.001). LVI was associated significantly with a higher risk for developing lymph-node metastasis (P = 0.004). Calculating the prognostic relevance, LVI presented as an independent prognostic parameter for disease free as well as overall survival (P = 0.001, and P = 0.001, respectively).
Conclusion:
Our data provide evidence that the biologic system of lymphangiogenesis constitutes a potential new target for development of anti-breast cancer therapeutic concepts. Our results further suggest that young, premenopausal patients with low differentiated breast tumors and high LMVD and LVI would, in particular, benefit from lymphangiogenesis-associated therapeutic strategies.
The prognostic relevance of lymphangiogenesis and lymphovascular invasion in breast cancer has not been investigated using a specific lymph-endothelial marker. Here we investigated the clinical relevance of lymphangiogenesis and lymphovascular invasion in 374 breast cancer patients using the lymph-endothelial specific marker podoplanin. Our data provide evidence that the system of lymphangiogenesis constitutes a potential new target for the development of anti-breast cancer therapeutic concepts.
The major cause of death from breast cancer is dissemination of the primary tumor leading to formation of metastases. Spread to axillary lymph nodes is often the first step of generalization.1 Thus, the presence of lymph node metastasis represents a major criterion for evaluating the potential prognosis of breast cancer patients and predicts the choice of additional chemotherapy and/or radiation therapy after surgery of primary tumor.2
Tumor-associated lymphatic vessels are considered as the main rout of tumor cells to axillary lymph nodes.3 Lymphovascular invasion (LVI) of tumor cells is a prerequisite for the dissemination via the lymphatic system. Tumor cells exposed to more microvessels are more likely to spread to distant sites and to lymph nodes.4,5 Therefore, antilymphangiogenic therapies have been suggested as novel therapeutic concepts, and first preliminary experimental studies show promising results.6
Studies of lymphatic vessels and lymphogenic metastasis have been hampered by the lack of specific lymphatic markers.7 Until recently, immunohistochemical identification of lymphatic vessels was, somehow unreliably, achieved by comparing staining of pan-endothelial markers like PECAM-1/CD318,9 with markers of the basal lamina, eg, collagen type IV.10 Vessels that reacted with CD31 but lacked a basement membrane staining with red blood cells in their lumens were deemed lymphatic.9 More recently, lymphatic vessel identification has been made possible by the identification of the vascular endothelial growth factor receptor-3 (VEGFR-3), which is predominantly expressed on lymphatic endothelium in normal adult tissue but is also up-regulated on blood vessel endothelium in tumors limiting its use in visualizing tumor-associated lymphangiogenesis.11,12 Until now, two studies about the clinical relevance of lymphangiogenesis in human breast cancer exist: Jacquemier et al using VEGFR-3 as lymphatic marker saw no association between their microvessel count and the patient's lymph node status or overall survival.13 The second study by Nathanson et al applying an immunohistochemical staining combination with factor VIII, collagen 4, and VEGFR-3 to identify lymphatic vessels, in contrast, described a significant correlation between high VEGFR-3-microvessel density and the patient's lymph node status.3 The predictive value of LVI, determined by the presence of neoplastic cell emboli within spaces showing a clear endothelial lining, is also being discussed controversially.14–16 The identification of podoplanin as a specific marker of the lymphatic endothelium and the development of a polyclonal antibody have enabled us to selectively stain lymphatic vessels.17 Therefore, to our knowledge, this study presents the first data correlating lymphangiogenesis and lymphovascular invasion on the prognosis in a representative cohort of breast cancer patients. Although respective, preliminary evidence does exist,3,13,15,18 the prognostic relevance of lymphangiogenesis and lymphovascular invasion in breast cancer has not yet been investigated in a large cohort using a specific lymphatic marker.
The aim of this study was the investigation of lymphangiogenesis and lymphovascular invasion, its predictive role of lymph node involvement, and its prognostic relevance in a large series of breast cancer with long-term follow-up.
MATERIALS AND METHODS
Patients and Tissues
The study population consisted of 374 randomly collected cases with invasive breast cancer UICC stages 1 to 4 that participated in one of five prospective, randomized, multicenter trials conducted by the Austrian Breast and Colorectal Cancer Study Group.19 All tumors were regraded according to Elston and Ellis.20 Special care was taken to include only specimens with sufficient amounts of normal tissue surrounding the invasive tumor.
All patients underwent breast surgery and dissection of axillary lymph nodes, containing at least 10 nodes.
Immunohistochemistry and Determination of Lymphatic Microvessel Density (LMVD)
Rabbit anti-human podoplanin IgG was raised against the recombinant human homologue of the rat 43-kDa glycoprotein podoplanin, as described previously.17 Affinity purification of rabbit serum was performed using nitrocellulose strips containing recombinant protein.21 Four-micrometer-thick serial sections of selected paraffin were cut, deparaffinized in xylol, rehydrated, and microwave pretreated in citrate buffer at 600 W for 10 minutes. After cooling for 15 minutes and washing in phosphate-buffered saline (PBS), endogenous peroxidase was blocked by using 3% hydrogen peroxide for 15 minutes, followed by incubation with PBS containing 10% normal goat serum for 30 minutes. For immunostaining of podoplanin, specimens were incubated at 20°C with the polyclonal rabbit antibody in a dilution of 1:200 for 1 hour. Detection of positive staining was performed using biotinylated goat anti-rabbit IgG or horse antimouse IgG (both Vector Laboratories, Burlingame, CA) for 30 minutes at 20°C followed by a streptavidin-peroxidase complex, according to the manufacturer's instructions. Peroxidase reaction product was visualized by diaminobenzidine (Serva, Heidelberg, Germany). Slides were counterstained with hematoxylin.
Determination of LMVD assessed by immunostaining for podoplanin was performed as suggested by Weidner et al.5,22 In brief, after scanning the immunostained section at low magnification (40×), the area of tissue with the greatest number of distinctly highlighted microvessels (“hot spot”) was selected. LMVD was then determined by counting all immunostained vessels at a total magnification of 200× corresponding to an examination area of 0.7386 mm2. Determination of the staining reaction was strictly confined to the hot spots. LVI was considered evident if at least one tumor cell cluster was clearly visible inside the podoplanin-stained vascular space.23 Microvessel counts were done by two independent observers, naive to the patient's pathologic and clinical status. The mean value of microvessel densities observed by both investigators in each patient was entered into further calculations. In the case of interobserver differences >30% in microvessel count, the respective slides were reinvestigated by both observers using a discussion microscope (evident in <10% of cases).
A block of cervical cancer used in a previous study served as positive control.24 For negative control, a slide was prepared from the same tissue block and a preimmune serum was used instead of the primary antibody.
Estrogen receptor density was determined using the dextran charcoal method from snap-frozen tumor samples as described previously.25 For definition of estrogen receptor positivity, cutoff values of >10 fmol/L were used.26
Immunofluorescence
Immunofluorescence was performed on acetone-fixed cryostat breast cancer sections with podoplanin sera produced in mice and LYVE-1 (affinity purified rabbit-anti IgG, kindly provided by Dr. David Jackson, Oxford, UK). Alexa 488 and Alexa 633 labeled secondary antibodies, and for nuclear counterstaining propidium iodide was used (all from Molecular Probes, Eugene, OR). Triple-channel confocal laser scanning microscopy analysis was performed on a Zeiss LSM 510.
Statistics
Association of LMVD and LVI with clinical and pathohistologic parameters was investigated using Kruskal-Wallis test or Spearman's coefficient of correlation, as appropriate. Overall survival (OS) was defined as the period from primary surgery until death of the patient. Death from a cause other than breast cancer, or survival until the end of the observation period, was considered a censoring event. Disease-free survival (DFS) was defined from the end of primary therapy until first evidence of progression of disease. Univariate analysis of OS and DFS was performed as outlined by Kaplan and Meier.27 The Cox proportional-hazard model was used for multivariate analysis. LMVD, LVI, histologic grading according to Elston and Ellis,20 patient's age, lymph node status, tumor stadium according to UICC, and histologic tumor type were entered into Cox regression. For all of the tests, a two-tailed P of ≤ 0.05 was considered significant.
RESULTS
Clinical Data
The mean patient's age at time of surgery was 57 years (median, 57.6 years); 124 patients (33.2%) were premenopausal, 240 (64.1%) postmenopausal, and in 10 (2.7%) the status was not known. Mean estrogen receptor density was 106.52 ± 173.29 fmol/L, and 288 (77%) were considered as estrogen-receptor positive, and 78 (20.9%) as estrogen receptor negative. For 8 patients (2.1%) the receptor status was not known.
As surgical treatment, breast conservation (usually wide excision) was performed in 133 patients (35.5%), and mastectomy in 205 (54.7%), including one patient treated with surgery after Rotter-Halstead. For 36 patients (9.6%) primary surgical treatment could not be evaluated. After breast conservation, the majority of patients were treated with adjuvant radiotherapy, except a small subgroup of patients with minimal risk. After surgery 55 patients (14.7%) received no adjuvant therapy. In 184 (49.1%) tamoxifen was administered for 5 years at a dose of 20 mg/day. Forty-three patients (11.5%) patients received a combined adjuvant chemotherapy (6 × CMF intravenously for 6 cycles, days 1 and 8, recycled on day 28, at the given doses: cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and fluorouracil 600 mg/m2). A combination of CMF plus tamoxifen was administered in 80 patients (21.4%). Four patient (1.1%) received tamoxifen plus Orimeten.
Eight patients (2.1%) received goserelin for 3 years (3.6 μg s.c. q 28 days) plus tamoxifen (29 mg/day) for 5 years. Most of patients were treated within prospective clinical trials and thus documentation and follow-up is excellent; 212 patients (56.6%) were staged lymph node negative whereas 162 patients (43.3%) had positive axillary lymph nodes. For staging at least 10 lymph nodes were examined. Tumor grading was as followed: 54 tumors (14.4%) were graded G1, 169 (45.1%) G2, and 151 carcinomas were graded G3 according to Elston; 207 breast tumors (55.3%) were staged pT1, 148 tumors (39.6%) pT2, 3 tumors (0.8%) pT3, and 16 tumors (4.3%) were staged pT4 according to UICC stages; and 327 patients (87.4%) had ductal NOS carcinomas and 47 patients (12.6%) had tumors histologic typed lobular carcinomas.
Immunofluorescence and Immunohistochemistry
Triple immunofluorescence confirmed that the grand majority of vessels (>97%) labeled with podoplanin also expressed LYVE-1 (Fig. 1).
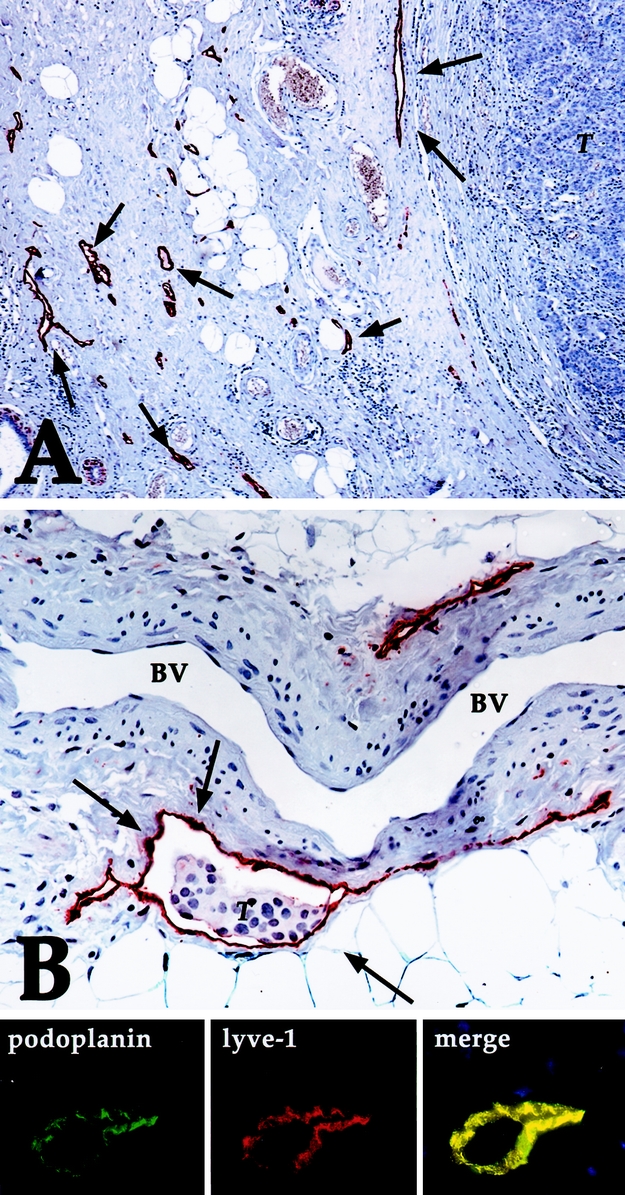
FIGURE 1. A: Breast cancer specimen with a high peritumoral LMVD (some of the lymphatic vessels stained for podoplanin are marked with arrows). Note the absence of lymphatic vessels in the tumor (T) (immunoperoxidase, original magnification ×200). B: Podoplanin-stained lymphatic vessel (arrows) with tumor cells (T) inside (LVI). Note the absence of endothelial podoplanin staining in a venous blood vessel (BV) with typical smooth muscle cells within its wall (immunoperoxidase, original magnification ×400). Line below shows immunofluorescence on lymphatic vessel for podoplanin and lyve-1, revealing perfect overlap (merge). Immunofluorescence, nuclear counterstaining with propidium iodide.
Intratumoral lymphatic vessels were extremely rare (observed in 5 cases), whereas most lymphatic vessels were located within the tumor stroma, at the border front to invasive tumor formations, as reported previously.18 Lymphangiogenesis carcinomatosa was mainly seen in open lymphatic vessels but was absent in narrow or collapsed lymphatic spaces (Fig. 1B).
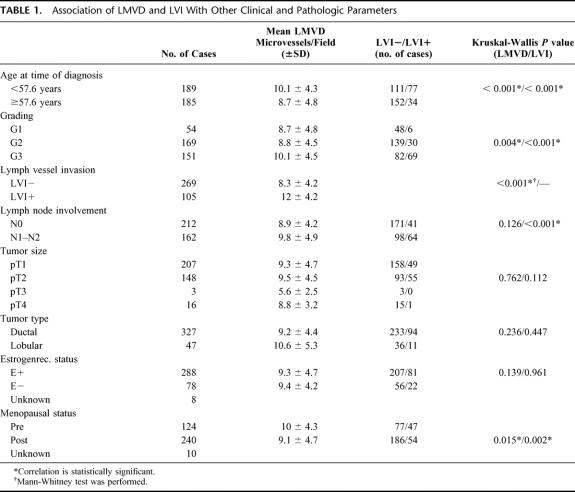
Median LMVD was 9.4 microvessels/ field (range 0–31 vessels). LVI was observed in 105 cases (28.1%). A significant correlation was seen between histologic grading and LMVD (P = 0.004, Mann-Whitney U test), between LMVD and LVI (P = 0.001, Mann-Whitney U test), and between LVI and the lymph node status (P < 0.001, Mann-Whitney U test). Young (< 57.6 years at time of surgery) and premenopausal patients had significantly higher LMVD (10.1 ± 4.3 vs. 8.7 ± 4.8, P < 0.001, and 10 ± 4.3 vs. 9.1 ± 4.7, P < 0.001 respectively, both Mann-Whitney U test) and presented significantly more often with LVI (41% vs. 18.3% of LVI+, P < 0.001, and 37.9% vs. 22.5% of LVI+, respectively, both Mann-Whitney U test) than older (> 57.6 years) and postmenopausal women. There was no significant correlation between the estrogen receptor status, tumor size, or histologic type of tumor and LMVD or LVI (Table 1).
TABLE 1. Association of LMVD and LVI With Other Clinical and Pathologic Parameters
Survival Analysis
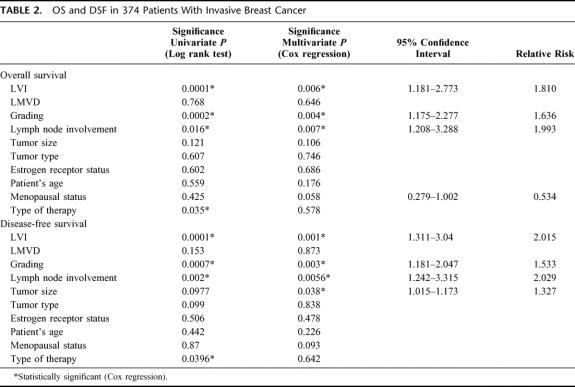
The mean observation time was 268.4 months (range, 8–510 months). In univariate survival analysis, a significant difference in OS and DFS was found between patients with or without LVI (P = 0.0001 and P = 0.0001, respectively, log-rank test [Fig. 2]), lymph node status (P = 0.016 and P = 0.002, respectively, log-rank test), histologic grading (P = 0.0002 and P = 0.0007, respectively, log-rank test), and type of therapy (P = 0.035 and P = 0.039, respectively, log-rank test). LMVD (P = 0.768 and P = 0.153 for OS and DFS, respectively, log-rank test) as well as the patient's age and other clinical and histopathologic parameters had no influence on OS and DFS in our collective.
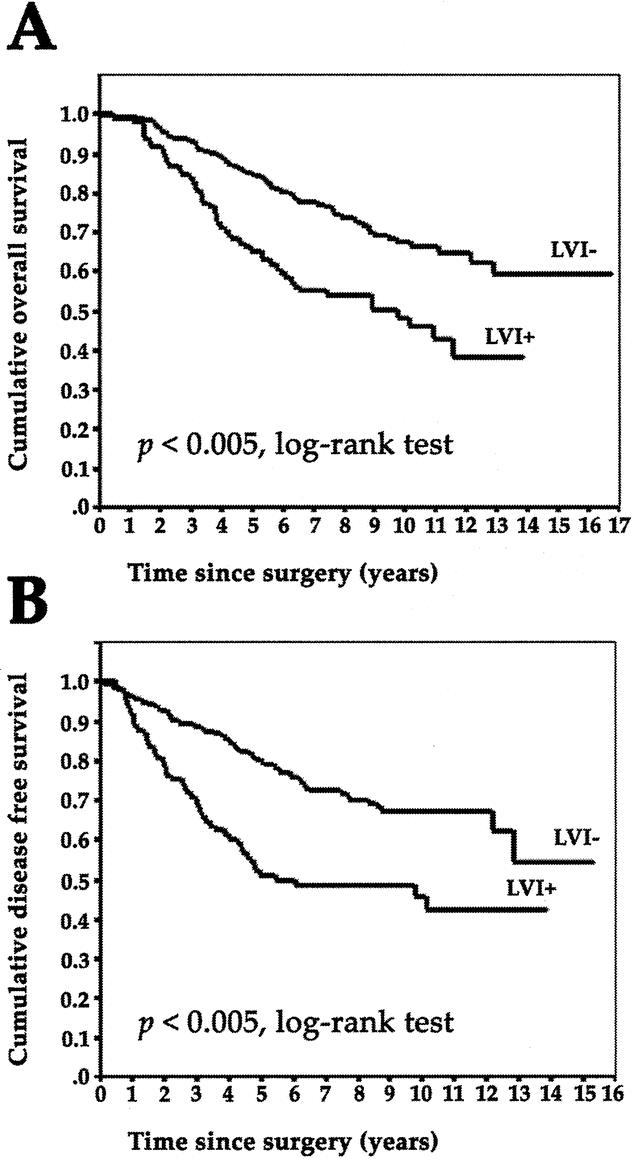
FIGURE 2. A: Overall survival in 374 breast cancer patients with (LVI+) or without (LVI-) lymphovascular invasion. B: Disease-free survival in 374 breast cancer patients with (LVI+) or without (LVI-) lymphovascular invasion.
LVI remained an independent prognostic factor for OS (P = 0.006) and DFS (P = 0.001) in multivariate analysis. Further histologic grading (P = 0.004 and P = 0.003, respectively, for OS and DFS, Cox regression), and lymph node involvement (P = 0.007 and P = 0.0056, respectively, for OS and DFS, Cox regression) remained independent prognostic factors. Tumor size was shown as significant prognostic factor in multivariate analysis only for DSF (P = 0.038, Cox regression) (Table 2).
TABLE 2. OS and DSF in 374 Patients With Invasive Breast Cancer
DISCUSSION
The invasion and metastasis of tumor cells are important biologic features of neoplasm and the main cause for poor prognosis and death.28 Axillary lymph node status at time of diagnosis is the most significant and durable prognostic factor in breast cancer patients.1 Various studies have been focused on the identification of characteristics of primary tumors predictive of lymph node involvement.29,30 In this study, we investigated the impact of lymphangiogenesis, presented in the number of lymphatic vessels surrounding the tumors, and the lymphovascular invasion in peritumoral lymphatic capillaries on the prognosis in invasive breast cancer using a lymphatic endothelial specific markers.17,31,32
Lymphatic vessels were almost exclusively found at the tumor's invasion front and not within the tumor formations, a finding that goes in good accordance with recently published studies.18,24,33,34 Lymph node metastases were shown to occur in tumors that lack intratumoral functional lymphatics, suggesting that functional lymphatics at the tumor margins are responsible for lymphatic dissemination.33 One hypothesis means that intratumoral lymphatic vessels collapse, caused by the physical stress exerted by the growing tumor making them unlikely to be some as the entrance for metastatic tumor cell spread.34 Here we show that breast cancers with high peritumoral lymphangiogenesis, measured with peritumoral LMVD, significantly more often invade these lymphatic vessels (lymphangiogenesis carcinomatosa). This significant association between LMVD and LVI could be attractively explained through a lymphangiogenesis-induced increase of the “lymphatic window” providing tumor cells with more opportunities to enter into lymphatic vessels. The risk of developing lymph node metastasis increases significantly with the presence of lymphangiogenesis carcinomatosa so lymphovascular invasion could likely be regarded as the precursor of nodal involvement. However, only LVI and not LMVD showed as a prognostic factor in our patient cohort, as well as for OS and DFS. Further, there was no significant association calculated between the degree of LMVD and the risk of patients to develop lymph node metastasis. Therefore, it is tempting to speculate that a sufficient number of peritumoral lymphatic vessel is a prerequisite for lymphovascular invasion by tumor cells, but one has also to keep in mind that additional, biologic mechanisms, involving multiple steps and molecular participants, are finally necessary for the tumor cell to invade lymphatic vessels.35,36
The significant association between the patient's age and LMVD and LVI goes in good correlation with studies that reported an independent association of patient's age and nodal involvement37 and might be explained by the fact that young age is also an independent prognostic factor for women with breast cancer.
High LMVD and great risk of LVI were associated with a low histologic differentiation grade, leading to the speculation that a fast-growing tumor produces more growth factors and offers a bigger clonal variety of tumor cells capable of invading lymphatic vessels compared with well-differentiated slow-growing tumors.
Whereas published data indicate that tumor cells can actively induce tumor-associated lymphangiogenesis and lymphatic metastasis by expressing the lymphangiogenic VEGF-C, a recent report indicates that lymphatic vessels might, in turn, actively promote tumor cell attraction and lymphatic metastasis.38 So the question remains if VEGF-C contributes to lymph node metastasis by boosting the number of lymphatic vessels or by promoting hyperplasia and dilation of peritumoral lymph vessels.39 The fact that we saw most LVI in open lymphatic vessels, and at the same time noticed a statistical significant association between LMVD and LVI, provides evidence that a combination of both effects might be necessary for a tumor to metastasize. Thus, it appears reasonable to hypothesize that blocking the growth and/or phenotype of newly formed lymphatic vessels could alter or finally inhibit lymphangiogenic metastases.
Our results also indicate the high predictive value of LVI and LMVD in breast cancer for a tumor's high metastatic potential. Surgeons and pathologists should be especially diligent and thorough in searching for lymph nodes in premenopausal, young patients with low differentiated tumors presenting with high LMVD and LVI.
The system of lymphangiogenesis represents a potential new target for development of anti-cancer strategies. Specific lymphatic endothelial markers, such as podoplanin, Prox-1, and LYVE-1, now provide sufficient tools to open researchers to much better opportunity to monitor the effect of therapeutic concepts on tumor lymphangiogenesis. Our data also suggest that especially young, premenopausal patients with low differentiated breast tumors would benefit from a future lymphangiogenesis-associated therapy.
ACKNOWLEDGMENTS
The authors thank Dr. Birner for statistical assistance.
Footnotes
Reprints: Sebastian F. Schoppmann, MD, Department of Surgery, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria. E-mail: sebastian.schoppman@meduniwien.ac.at.
REFERENCES
- 1.Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997;47:28–51. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983;52:1551–1557. [DOI] [PubMed] [Google Scholar]
- 3.Nathanson SD, Zarbo RJ, Wachna DL, et al. Microvessels that predict axillary lymph node metastases in patients with breast cancer. Arch Surg. 2000;135:586–593; discussion 593–594. [DOI] [PubMed]
- 4.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. [DOI] [PubMed] [Google Scholar]
- 6.Schoppmann SF, Horvat R, Birner P. Lymphatic vessels and lymphangiogenesis in female cancer: mechanisms, clinical impact and possible implications for anti-lymphangiogenic therapies [Review]. Oncol Rep. 2002;9:455–460. [PubMed] [Google Scholar]
- 7.Sleeman JP, Krishnan J, Kirkin V, et al. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech. 2001;55:61–69. [DOI] [PubMed] [Google Scholar]
- 8.Newman PJ, Berndt MC, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. [DOI] [PubMed] [Google Scholar]
- 9.Lymboussaki A, Partanen TA, Olofsson B, et al. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol. 1998;153:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nerlich AG, Schleicher E. Identification of lymph and blood capillaries by immunohistochemical staining for various basement membrane components. Histochemistry. 1991;96:449–453. [DOI] [PubMed] [Google Scholar]
- 11.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- 13.Jacquemier J, Mathoulin-Portier MP, Valtola R, et al. Prognosis of breast-carcinoma lymphangiogenesis evaluated by immunohistochemical investigation of vascular-endothelial-growth-factor receptor 3. Int J Cancer. 2000;89:69–73. [DOI] [PubMed] [Google Scholar]
- 14.Woo CS, Silberman H, Nakamura SK, et al. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg. 2002;184:337–340. [DOI] [PubMed] [Google Scholar]
- 15.Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann Surg. 1999;230:692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Kimura T, Miyakawa R, et al. Clinicopathologic study associated with long-term survival in Japanese patients with node-negative breast cancer. Br J Cancer. 2000;82:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoppmann SF, Birner P, Studer P, et al. Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer. Anticancer Res. 2001;21:2351–2355. [PubMed] [Google Scholar]
- 19.Jakesz R, Samonigg H, Gnant M, et al. Significant increase in breast conservation in 16 years of trials conducted by the Austrian Breast & Colorectal Cancer Study Group. Ann Surg. 2003;237:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46:189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvat R, Hovorka A, Dekan G, et al. Endothelial cell membranes contain podocalyxin–the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986;102:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. [DOI] [PubMed] [Google Scholar]
- 23.Heimburg S, Oehler MK, Papadopoulos T, et al. Prognostic relevance of the endothelial marker CD 34 in ovarian cancer. Anticancer Res. 1999;19:2527–2529. [PubMed] [Google Scholar]
- 24.Schoppmann SF, Schindl M, Breiteneder-Geleff S, et al. Inflammatory stromal reaction correlates with lymphatic microvessel density in early-stage cervical cancer. Anticancer Res. 2001;21:3419–3423. [PubMed] [Google Scholar]
- 25.Jakesz R, Dittrich C, Hanusch J, et al. Simultaneous and sequential determinations of steroid hormone receptors in human breast cancer: influence of intervening therapy. Ann Surg. 1985;201:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakesz R, Hausmaninger H, Haider K, et al. Randomized trial of low-dose chemotherapy added to tamoxifen in patients with receptor-positive and lymph node-positive breast cancer. J Clin Oncol. 1999;17:1701–1709. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Statist Assoc. 1985;53:457–481. [Google Scholar]
- 28.Thames HD, Buchholz TA, Smith CD. Frequency of first metastatic events in breast cancer: implications for sequencing of systemic and local-regional treatment. J Clin Oncol. 1999;17:2649–2658. [DOI] [PubMed] [Google Scholar]
- 29.Ferno M. Prognostic factors in breast cancer: a brief review. Anticancer Res. 1998;18:2167–2171. [PubMed] [Google Scholar]
- 30.Schnitt SJ. Traditional and newer pathologic factors. J Natl Cancer Inst Monogr. 2001;30:22–26. [DOI] [PubMed] [Google Scholar]
- 31.Breiteneder-Geleff S, Soleiman A, Horvat R, et al. [Podoplanin–a specific marker for lymphatic endothelium expressed in angiosarcoma]. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- 32.Kriehuber E, Breiteneder-Geleff S, Groeger M, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. [DOI] [PubMed] [Google Scholar]
- 34.Leu AJ, Berk DA, Lymboussaki A, et al. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 35.Auvinen P, Tammi R, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol. 2000;156:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani H. Stromal reaction in cancer tissue: pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol Int. 1998;48:1–9. [DOI] [PubMed] [Google Scholar]
- 37.Fein DA, Fowble BL, Hanlon AL, et al. Identification of women with T1-T2 breast cancer at low risk of positive axillary nodes. J Surg Oncol. 1997;65:34–39. [DOI] [PubMed] [Google Scholar]
- 38.Wiley HE, Gonzalez EB, Maki W, et al. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. [DOI] [PubMed] [Google Scholar]
- 39.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. [DOI] [PubMed] [Google Scholar]