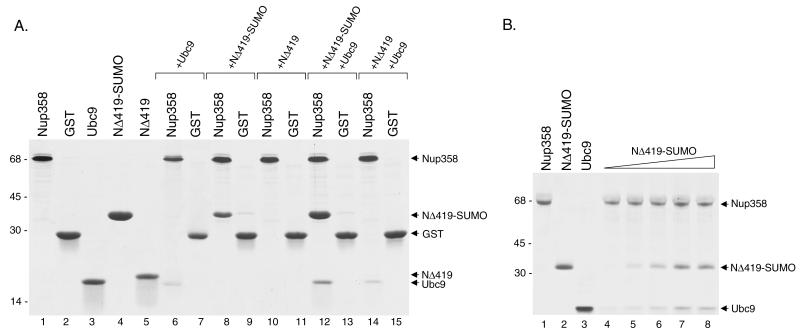
FIG. 8.
Ubc9 and SUMO-1-modified RanGAP1 both interact directly with Nup358 to form a trimeric complex. (A) A carboxyl-terminal region of Nup358 (amino acids 2596 to 2836) was expressed as a GST fusion protein in bacteria and purified (lane 1). Other proteins, including GST (lane 2), Ubc9 (lane 3), and the carboxyl-terminal domain of RanGAP1 (NΔ419) (lane 5), were also expressed in bacteria and purified. NΔ419 was modified by SUMO-1 in an in vitro assay and purified from reaction components (lane 4). To assay for interactions with Nup358, the GST-Nup358 fusion protein was immobilized on glutathione-Sepharose beads and incubated with Ubc9 alone (lane 6), SUMO-1-modified NΔ419 (NΔ419-SUMO) alone (lane 8), NΔ419 alone (lane 10), NΔ419-SUMO plus Ubc9 (lane 12), and NΔ419 plus Ubc9 (lane 14). Following washes, bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE. As controls for binding specificity, similar binding assays were also performed with glutathione-Sepharose beads containing GST alone (lanes 7, 9, 11, 13, and 15). Molecular mass standards (kilodaltons) are indicated on the left. (B) The synergistic effect of SUMO-1-modified RanGAP1 on the binding of Ubc9 to Nup358 was investigated. A fixed amount of Ubc9 (5 μg) was incubated with immobilized Nup358 in the presence of increasing amounts of SUMO-1-modified RanGAP1 (lane 4, 0 μg; lane 5, 1 μg; lane 6, 2 μg; lane 7, 4 μg; and lane 8, 8 μg).