Abstract
Objective:
To determine whether pancreatic digestive enzymes released into the ischemic gut during an episode of T/HS are involved in the generation of distant organ injury. This hypothesis was tested by examining the effect of PDL on T/HS-induced intestinal injury, lung injury, and RBC deformability.
Summary Background Data:
The effect of pancreatic duct ligation (PDL) on distant organ injury following trauma/hemorrhagic shock (T/HS) was examined. PDL before T/HS decreases lung and red blood cell (RBC) injury and exerts a limited protective effect on the gut. Pancreatic proteases in the ischemic gut appear to be involved in gut-induced lung and RBC injury. Based on recent work, it appears that proinflammatory and/or toxic factors, which are generated by the ischemic intestine, play an important role in the pathogenesis of multiple organ failure. The process by which these toxic factors are generated remains unknown. Previous experimental work has clearly documented that intraluminal inhibition of pancreatic proteases decreases the degree of T/HS-induced lung injury and neutrophil activation. One possible explanation for this observation is that the toxic factors present in intestinal lymph are byproducts of interactions between pancreatic proteases and the ischemic gut.
Methods:
Male Sprague-Dawley rats were subjected to a laparotomy (trauma) and 90 minutes of sham (T/SS) or T/HS with or without PDL. At 3 and 24 hours following resuscitation, animals were killed and samples of gut, lung, and blood were collected for analysis. Lung permeability, pulmonary myeloperoxidase levels, and bronchoalveolar fluid protein content were used to quantitate lung injury. Intestinal injury was determined by histologic analysis of terminal ileum (% villi injured). To assess RBC injury, RBC deformability was measured, as the RBC elongation index (RBC-EI), using a LORCA device.
Results:
At 3 and 24 hours following resuscitation, PDL prevented shock-induced increases in lung permeability to both Evans blue dye and protein in addition to preventing an increase in pulmonary myeloperoxidase levels. T/HS-induced impairments in RBC deformability were significantly reduced at both time points in the PDL + T/HS group, but deformability did not return to T/SS levels. PDL did reduce the magnitude of ileal injury at 3 hours after T/HS, but the protective effect was lost at 24 hours after T/HS.
Conclusions:
PDL prior to T/HS decreases lung injury and improves RBC deformability but exerts a limited protective effect on the gut. Thus, the presence of pancreatic digestive enzymes in the ischemic gut appears to be involved in gut-induced lung and RBC injury.
The present study tested whether pancreatic digestive enzymes produced during an episode of T/HS contribute to T/HS-induced gut and lung injury as well as RBC dysfunction. The results of this study, documenting that ligation of the pancreatic duct prevents shock-induced lung injury, support this hypothesis. Clearly, the pancreas appears to be playing an important role in the development of distant organ injury and subsequent failure following T/HS. Further studies will be required to elucidate the mechanisms and factors involved in this process.
Gut barrier failure, with the ensuing translocation of bacteria and endotoxin from the gut, has been proposed as a major contributor to the development of systemic infection and multiple organ failure following shock. Indeed, over the past decade, both clinical and experimental studies have implicated gut injury with the development of an exaggerated systemic inflammatory state and distant organ injury.1 However, the absence of detectable bacteremia or endotoxemia in the portal blood of trauma victims has cast doubt on the role of the gut in the pathogenesis of multiple organ failure. Yet, based on recent work, it appears that proinflammatory and/or toxic factors, which are generated by the ischemic intestine, are reaching the systemic circulation via the mesenteric lymphatics rather than by the portal venous route.2 These factors carried in intestinal lymph have been shown to induce a wide range of physiologic effects, including endothelial cell death and dysfunction as well as neutrophil activation and up-regulation of adhesion molecule expression.3–5 Nevertheless, the process by which these toxic factors are generated remains unknown. Previous work from our and other laboratories has shown that intraluminal inhibition of pancreatic proteases decreases the degree of trauma/hemorrhagic shock (T/HS)-induced lung injury and neutrophil activation.6,7 One possible explanation for this observation is that the toxic factors present in intestinal lymph are byproducts of interactions between pancreatic proteases and the ischemic gut. In this context, we tested the hypothesis that pancreatic digestive enzymes released into the gut after T/HS are involved in the generation of toxic and/or biologically active factors contained in mesenteric lymph. This hypothesis was tested by determining whether inhibition of pancreatic enzyme activity, by ligation of the pancreatic duct, reduces shock-induced gut and lung injury as well as red blood cell (RBC) dysfunction.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY), weighing 300 to 410 g, were housed under barrier-sustained conditions and kept at 25°C with 12-hour light/dark cycles. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, WI). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the New Jersey Medical School Animal Care Committee.
Experimental Design
The major goal of this study was to test the hypothesis that pancreatic digestive enzymes produced during an episode of T/HS contribute to T/HS-induced gut and lung injury as well as RBC dysfunction. To accomplish this goal, the effect of pancreatic duct ligation prior to trauma and hemorrhagic shock on subsequent gut injury, lung injury, and RBC deformability was tested. Animals were divided into 4 groups: trauma-sham shock (T/SS), T/HS, pancreatic duct ligation (PDL) + T/SS, and PDL + T/HS.
In the T/HS group, animals underwent laparotomy followed by 90 minutes of hemorrhagic shock. In the PDL + T/HS group, animals underwent laparotomy plus PDL and 90 minutes of hemorrhagic shock. In these animals, the main pancreatic duct was ligated immediately prior to the induction of hemorrhagic shock. Two control groups were included. In the T/SS group, animals underwent cannulation of vessels and laparotomy but were not subjected to hemorrhagic shock. In the PDL + T/SS group, animals underwent cannulation of vessels and laparotomy plus PDL but were not subjected to hemorrhagic shock. All shocked animals were resuscitated with their shed blood. At 3 and 24 hours following resuscitation, animals were killed and lung permeability was measured. In addition, lung samples were obtained for quantitating pulmonary leukosequestration, and a specimen of the ileum was obtained for histologic study. Blood samples (0.1 mL) were taken preshock, preresuscitation, and 1, 3, and 24 hours postresuscitation for RBC deformability analysis.
The 3-hour time point was selected based on previous studies, which showed that lung injury occurs in this period using this model.8 An additional 24-hour time point was chosen to ensure that the results obtained were not transient in nature.
Surgical Procedure
Rats were weighed and anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). Using aseptic techniques, the internal jugular vein and femoral artery were isolated and cannulated with polyethylene (PE-50) tubing or 50-gauge silicone catheter containing 0.1 mL heparinized saline (10 units/mL), respectively. Both catheters (internal jugular vein and femoral artery) remained in situ for the duration of the experiment. Next, a 3-cm midline laparotomy (trauma) was performed followed by either closure with a running 3–0 silk suture or pancreatic duct ligation prior to closure. The main pancreatic duct was identified in the mesentery of the duodenum and ligated with a 4–0 silk suture prior to division. At necropsy, the pancreas in the duct-ligated group did not appear to be hemorrhagic or edematous on gross examination; thus, there was no gross evidence of duct ligation-induced pancreatitis.
Next, the femoral artery catheter was attached in line to a blood-pressure monitor (BP-2 Digital Blood Pressure Monitor, Columbus Instruments, Columbus, OH) for continuous blood pressure monitoring. Blood was then withdrawn from the internal jugular vein. The mean arterial pressure was reduced to 30 mm Hg and maintained at this level for 90 minutes by withdrawing or reinfusing shed blood (kept at 37°C) as needed. Rectal temperature was monitored throughout the shock period and maintained at approximately 37°C by using an electric heating pad under the surgical platform. At the end of the shock period, animals were resuscitated by reinfusing all the shed blood at 1 mL/min. In this model, the mean arterial pressure returns to normal within a few minutes of the animals receiving their blood back and remains there for the duration of the experiment. The long-term mortality rate ranges from 14% to 25% with most deaths occurring during or just after the shock period.9 The T/SS rats were anesthetized and their vessel cannulated, but no blood was withdrawn or infused. All animals remain anesthetized throughout the duration of the experiment.
Lung Permeability
Lung permeability was measured by both the EBD and by determining the bronchoalveolar fluid (BALF) to plasma protein ratio as previously described.2 At 3 hours following the resuscitation period, animals were injected with 10 mg of EBD through the jugular vein catheter. The EBD was then allowed to circulate for 5 minutes, at which time a blood sample was withdrawn (1.0 mL) via the femoral artery catheter to determine plasma EBD concentration. Twenty minutes after the injection of dye, the animals were killed and the lungs were harvested. Bronchoalveolar lavage was performed on the excised lungs by lavaging 3 times with 5-mL aliquots of normal saline. The recovered BALF was then centrifuged at 1500 rpm at 4°C for 10 minutes. The supernatant fluid and the plasma were then assayed spectrophotometrically at 620 nm to measure the concentration of EBD. The concentration of EBD in the BALF was then expressed as the percentage of that in plasma.
The total protein content (g/dL) of both BALF and plasma collected from each animal was determined using a hand-held refractometer (Milton Roy, NY). Using these data, the BALF to plasma protein ratio was determined.3
Pulmonary Leukosequestration
Pulmonary neutrophil sequestration was quantified as myeloperoxidase (MPO) activity as previously described.2 Previously frozen lung samples were homogenized for 30 seconds in 4 mL of 20 mM potassium phosphate buffer, pH 7.4, and the centrifuged for 30 minutes at 40,000g at 4°C. The pellet was then resuspended in 4 mL of 50 mM potassium phosphate buffer, pH 6, containing 0.5 g/dL hexadecyltrimethyl ammonium bromide. Samples were then sonicated for 90 seconds at full power, followed by incubation for 2 hours in a 60°C water bath. Samples were then centrifuged and 100 microliters of the supernatant were added to 2.9 mL of 50 mM potassium phosphate buffer, pH 6, containing 0.167 mg/ml O-dianisidine and 0.0005% hydrogen peroxide. Absorbance at 460 nm of visible light (A460) was measured for 3 minutes. MPO activity per gram lung tissue was calculated as MPO activity (units/g tissue) = (δA460 × 13.5)/weight (g), where δA460 equals the rate of change in absorbance at 460nm between 1 and 3 minutes. One unit of MPO activity is the amount of enzyme that will reduce 1 μm peroxide per minute.
Histologic Analysis of the Ileum
At death, a segment of the terminal ileum was excised and fixed in 10% buffered formalin. After processing, semi-thin (2–4 μm) sections were cut and stained with toluidine blue. Five random fields with 100 to 250 villi from each animal were then analyzed in a blinded fashion using light microscopy at 100× magnification as previously described.10 The overall villous damage was expressed as a percentage of the number of injured villi to the total number of villi examined.
Measurement of RBC Deformability by Ektacytometry
RBC deformability was determined at various shear stresses by laser diffraction analysis, using an ektacytometer (LORCA; RR Mechtronics, Hoorn, The Netherlands) as previously described.11 The instrument consisted of a laser diode, thermostated bob-cup measuring system, stepper motor, and a video camera attached to a microcomputer. The microprocessor controlled the speed of the stepper motor, thus the rotational speed of the cup to generate various shear stresses in the dilute blood. For the study, 15 μL of each sample were suspended in 3 mL of a highly viscous solution of 360,000 MW polyvinylpyrrolidone in an isotonic phosphate buffer base (pH 7.4) and gently rotated at room temperature for 10 minutes to insure complete oxygenation of the hemoglobin. The samples were placed in a concentric-cylinder system made of glass, with a gap of 0.3 mm between the cylinders, and rotated at a shear stress of 0.3 Pa. The laser beam projected through the sample and the diffraction pattern produced by RBC was analyzed by the microcomputer. RBC elongation index was calculated by dividing the difference between the long and short axes by the sum of the 2 axes: A − B/A + B. The higher the elongation index, the more deformable are the RBCs.
Statistical Analysis
Results are expressed as mean ± SD. Continuous data was analyzed by one-way analysis of variance using the post hoc Neuman-Keuls test. Statistical significance was considered to be achieved at P < 0.05.
RESULTS
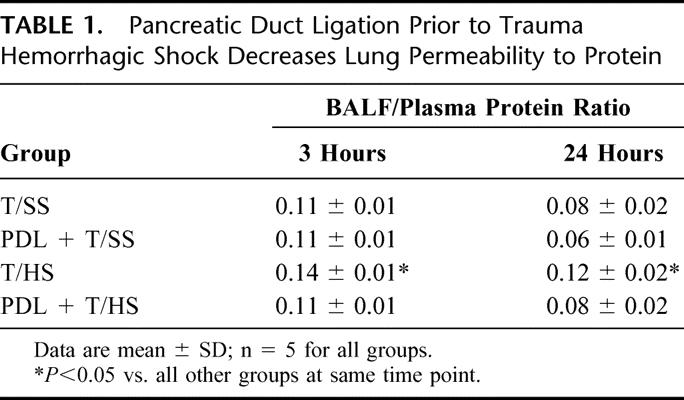
Lung permeability to EBD was greatest in those rats subjected to 90 minutes of hemorrhagic shock at both 3 and 24 hours. Ligation of the pancreatic duct before the hemorrhagic shock episode completely prevented this increase in pulmonary permeability (Fig. 1). After T/HS, there was also a significant increase in the BALF/plasma protein ratio at both 3 and 24 hours (Table 1). Similarly, PDL prior to the shock episode completely prevented the shock-induced increase in BALF protein content. Lung injury following PDL alone (PDL+T/SS), in the absence of T/HS, was not significantly different from the T/SS rats.
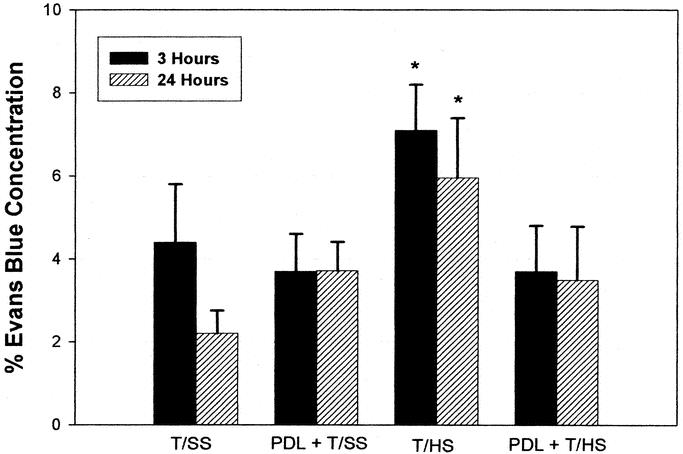
FIGURE 1. PDL prevents T/HS-induced increases in lung permeability at both 3 and 24 hours postshock. Data are mean ± SD. All groups, n = 6, except T/SS at 3 hours, n = 7. *P < 0.05 versus all other groups at 3 or 24 hours.
TABLE 1. Pancreatic Duct Ligation Prior to Trauma Hemorrhagic Shock Decreases Lung Permeability to Protein
Because MPO levels are a reliable and quantitative marker for neutrophil accumulation in tissues, pulmonary MPO levels were used to assess the degree of pulmonary neutrophil infiltration. Lung MPO levels were significantly higher in the T/HS rats compared with the sham-shocked rats at both 3 and 24 hours (Fig. 2). PDL prior to the shock period reduced pulmonary MPO accumulation at both 3 and 24 hours. Pulmonary MPO levels following PDL alone, in the absence of T/HS, were not significantly different from the nonshocked T/SS animals (Fig. 2).
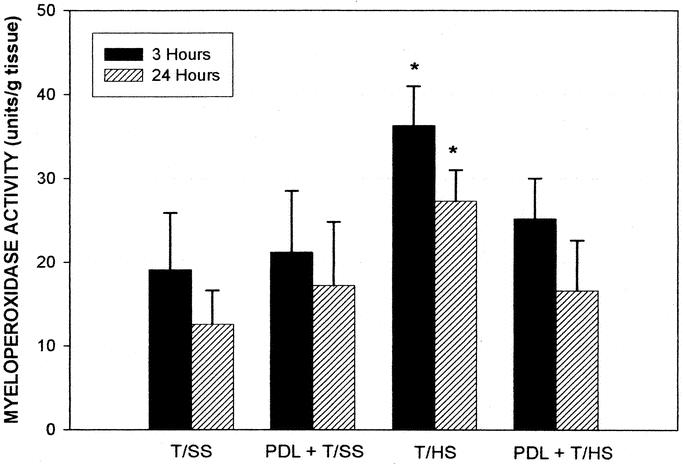
FIGURE 2. PDL prevents T/HS-induced increases in pulmonary leukosequestration. Data are mean ± SD; n = 6 in each group except for T/SS at 3 hours, n = 7. *P < 0.05 versus all other groups at 3 or 24 hours.
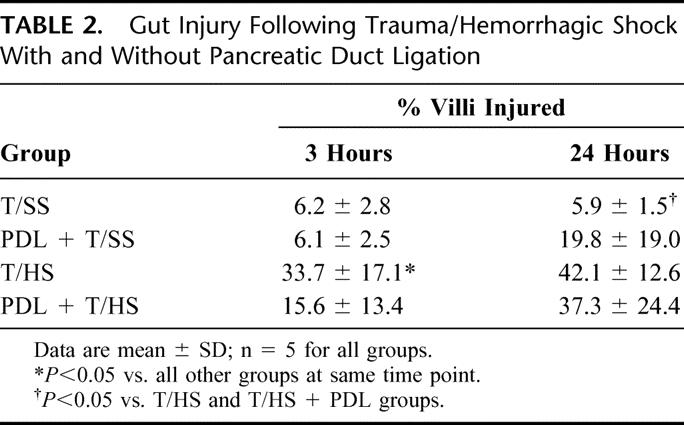
T/HS produced a predictable pattern of intestinal mucosal injury (Table 2). PDL provided a modest level of protection (15.6 ± 13.4% vs. 33.7 ± 17.1%) from this intestinal injury at 3 hours postresuscitation (P < 0.05) when compared with T/HS alone. However, at 24 hours following resuscitation, there was no significant difference in the percent of villi injured in those animals undergoing T/HS with or without PDL (Table 2).
TABLE 2. Gut Injury Following Trauma/Hemorrhagic Shock With and Without Pancreatic Duct Ligation
The T/HS-induced decrease in RBC deformability was reduced by PDL, and this protective effect of PDL was observed at both 3 and 24 hours after T/HS (Fig. 3). However, the RBC deformability of the PDL + T/HS rats was significantly less than that observed in the T/SS group, indicating that PDL ameliorated but did not completely prevent T/HS-induced loss of RBC deformability.
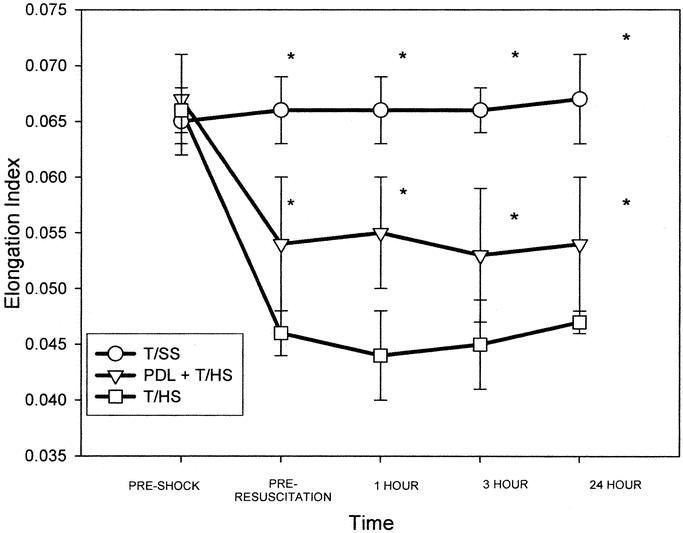
FIGURE 3. T/HS caused a significant decrease in RBC deformability, which was present at the end of the shock period prior to reperfusion and persisted for 24 hours. PDL ameliorated, but did not completely restore, the T/HS-induced decrease in RBC deformability to baseline or T/SS levels. Data are mean ± SD elongation index (EI) at shear stress 0.30 Pascals. N = 4 to 6 rats for each group at each time point. *P < 0.05 versus other groups at same time point.
DISCUSSION
Clinically and experimentally, severe pancreatitis has long been known to be a cause of acute lung injury and multiple organ dysfunction syndrome (MODS).1,12,13 Indeed, more than 30 years ago, it was proposed that the pancreas played an important pathologic role in the development of organ failure observed after hemorrhagic or traumatic shock.14–16 For example, hemorrhagic shock has been shown to stimulate the release of myocardial depressant factor from damaged pancreatic acinar cells,13 which in turn induces profound systemic hemodynamic changes.17 In addition to myocardial depressant factor, it has been suggested that other “shock factors” are also released from the ischemic pancreas, which, in turn, produce an exaggerated hypotensive response in targeted organ systems, including the brain and gut.18 More recently, work from the laboratory of Schmid-Schonbein, using a rat splanchnic arterial ischemia model provided evidence that pancreatic enzymes acting on the ischemic intestine are responsible for the production of factors leading to hypotension, neutrophil activation, and pulmonary injury.6,19 Since then, results from both Schmid-Schonbein's and our laboratory have documented that the administration serine pancreatic protease inhibitors into the lumen of the gut decreases lung injury, gut injury, and neutrophil activation in models of splanchnic artery occlusion20,21 and trauma-hemorrhagic shock.7 However, interpretation of the action of these pancreatic protease inhibitors is confounded by their ability to inhibit nonpancreatic as well as pancreatic serine proteases. For example, in addition to inhibiting the pancreatic serine proteases, trypsin and chymotrypsin, these agents also have been documented to inhibit complement proteins, thrombin, plasmin, kallikrein, lipase, as well as phospholipase A2.7 Consequently, to test the hypothesis that pancreatic enzymes are necessary for or contribute to distant organ injury in the absence of the potentially confounding effects associated with pharmacologic inhibition studies, we measured the effect of pancreatic duct ligation on T/HS-induced lung injury and RBC dysfunction as well as intestinal injury. Consequently, our results documenting that pancreatic duct ligation prevents T/HS-induced lung injury and ameliorates T/HS-induced RBC dysfunction provide further support for the hypothesis that pancreatic digestive enzymes interact with the ischemic intestine to generate factors that contribute to distant organ and cellular dysfunction.
The mechanisms by which pancreatic enzymes interacting with the ischemic gut result in the generation of biologically active factors that contribute to lung and RBC injury remain to be determined. However, under normal circumstances, the intact intestinal mucosal barrier prevents pancreatic enzymes within the intestinal lumen from reaching the submucosal space, where they could exert their enzymatic activities. In contrast, during periods of gut ischemia, as is observed during T/HS and other conditions, gut barrier function fails and intestinal permeability is increased.1 Not only has increased gut permeability been reported in animal models of T/HS,22 it has also been reported in patients after burn injury,23 major trauma,24 in ICU patients,25 as well as in patients undergoing major abdominal26 and vascular27 surgery. This notion is consistent with studies documenting that incubation of substimulatory concentrations of pancreatic homogenates with intestinal tissue resulted in the intestinal tissue extract manifesting toxic activities when injected into rats.19 Thus, one possible explanation for our results is that following a period of ischemia/reperfusion the intestinal protective barrier is weakened, allowing the pancreatic proteases to exert their effect on or within the wall of the intestine.
However, in trying to interpret the role of pancreatic enzymes in the pathogenesis of organ damage after T/HS, it is important to point out that the protective effect of pancreatic duct ligation varied based on the parameter being measured. That is, pancreatic duct ligation completely prevented T/HS-induced lung injury and pulmonary neutrophil sequestration, ameliorated RBC injury, and yet had only a modest and transient protective effect on gut injury. Indeed, our failure to find a significant protective effect of pancreatic duct ligation on T/HS-induced gut injury at 24 hours after T/HS differs from our previous studies in which perfusion of the intestinal lumen with the pancreatic protease inhibitor, nafamostat, largely prevented T/HS-induced gut injury.7 Furthermore, our previous observation that intestinal lavage with nafamostat prevented T/HS-induced gut injury is consistent with the studies of others showing that intestinal lavage with pancreatic protease inhibitors decreases intestinal ischemic damage.20,21 One explanation for these discordant findings would be that intestinal lavage washes out any pancreatic enzymes contained in the gut as well as other luminal factors, such as bacteria and bacterial products. This would not be the case with pancreatic duct ligation, since pancreatic enzymes present with the lumen of the gut prior to pancreatic duct ligation would still be present after duct ligation. Thus, it is possible that residual pancreatic enzymes within the gut lumen contributed to gut injury in the present study. Additionally, since intestinal lavage removes both bacteria and their products, it is also possible that lavage-related reductions in the gut flora had a protective effect on the gut. This possibility is supported by studies documenting that alterations in the gut flora modulate the cytokine response to hemorrhagic shock.28 It is also possible that dissolved oxygen within the lavage fluid itself could limit the degree of gut ischemia and thereby reduce the magnitude of gut injury.29 Further work will need to be done to resolve this issue. Nonetheless, the observation that T/HS-induced lung injury was prevented and RBC dysfunction was ameliorated by pancreatic duct ligation, even in the presence of gut injury, strongly implicates pancreatic digestive enzymes as playing an important role in the pathogenesis of gut-induced distant organ dysfunction.
Although the role of the gut and splanchnic organs in T/HS-induced RBC dysfunction has been less well studied than lung injury, the observation that pancreatic duct ligation reduces T/HS-induced RBC rigidification is consistent with our recent work indicating that division of the main mesenteric lymph duct prevents T/HS-induced decreases in RBC deformability.30 Since T/HS-induced impairment in RBC deformability appears to be caused by factors contained in intestinal lymph exiting the ischemic gut,30 it appears that pancreatic enzymes are involved, at least to some extent, in the generation of these RBC injurious factors. Loss of RBC deformability after T/HS is likely to be of physiologic significance because the increased rigidity seen following T/HS decreases the ease by which RBCs traverse the microcirculation, thereby interfering with oxygen delivery and potentiating shock-induced injury.
CONCLUSION
The results of the current study support the notion of a “multihit” hypothesis of distant organ injury following T/HS (Fig. 4). The pathophysiology of this process would entail ischemic injury of the intestine (first hit), followed by reperfusion injury during resuscitation (second hit), culminating in the creation of toxic factors via the interaction of pancreatic proteases with the ischemic intestine (third hit). This process can be further exacerbated by the translocation of bacteria and their products from the intestinal lumen into the gut wall, where they can exacerbate the inflammatory response (fourth hit). Since mechanical inhibition of pancreatic secretions by ligation of the pancreatic duct reduces T/HS-induced lung injury and RBC dysfunction, the results of this study suggest that pancreatic secretions play a role in the pathogenesis of shock-induced distant organ injury and appear to contribute to the generation of biologically active mesenteric lymph, most likely through an interaction with a primed and susceptible ischemic gut.
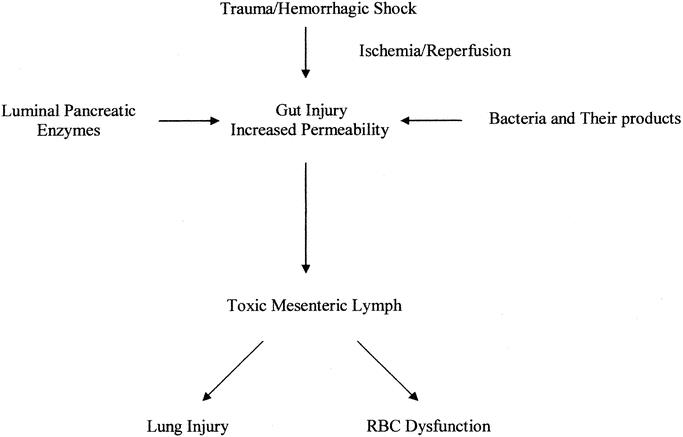
FIGURE 4. “Multihit” hypothesis of distant organ injury following T/HS. The pathophysiology of this process involves an ischemic injury of the intestine (first hit) followed by reperfusion injury during resuscitation (second hit), culminating in the generation of toxic factors via the interaction of pancreatic proteases with the ischemic intestine (third hit). This process is further fueled by the translocation of bacteria and their products from the intestinal lumen into the gut wall.
Footnotes
Supported in part by DOD Grant N00014-00-1-0878 (E.A.D.) and NIH Grant GM 59841 (E.A.D.).
Reprints: Edwin A. Deitch, MD, Department of Surgery, MSB G506, UMDNJ–New Jersey Medical School, 185 South Orange Avenue, Newark, NJ 07103. E-mail: edeitch@umdnj.edu.
REFERENCES
- 1.Deitch EA. Multiple organ failure: pathophysiology and potential future. Ann Surg. 1992;216:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnotti LJ, Upperman JS, Xu D, et al. Gut derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Upperman JS, Deitch EA, Guo W, et al. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407–414. [DOI] [PubMed] [Google Scholar]
- 4.Adams CA, Adams JM, Fekete Z, et al. Trauma-hemorrhage induced neutrophil activation is prevented by mesenteric lymph duct ligation. Shock. 2002;18:513–517. [DOI] [PubMed] [Google Scholar]
- 5.Zallen G, Moore EE, Johnson JL, et al. Post-hemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–88. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deitch EA, Shi HP, Lu Q, et al. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA, Adams C, Lu Q, et al. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. [DOI] [PubMed] [Google Scholar]
- 9.Baker JW, Deitch EA, Li M, et al. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896–906. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki Y, Deitch EA, Mishima S, et al. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia reperfusion injury. Crit Care Med. 2000;28:3692–3696. [DOI] [PubMed] [Google Scholar]
- 11.Zaets SB, Berezina TL, Morgan C, et al. Effect of trauma-hemorrhagic shock on red blood cell deformability and shape. Shock. 2002;19:268–273. [DOI] [PubMed] [Google Scholar]
- 12.Guice KS, Oldham KT, Johnson KJ, et al. Pancreatitis-induced acute lung injury: an ARDS model. Ann Surg. 1988;208:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwig W, Carter EA, Jiminez RE, et al. Neutrophil metabolic activity but not neutrophil sequestration reflects the development of pancreatitis-associated lung injury. Crit Care Med. 2002;30:2075–2082. [DOI] [PubMed] [Google Scholar]
- 14.Bounous G, Brown RA, Mulder DS, et al. Abolition of ‘tryptic enteritis’ in the shocked dog. Arch Surg. 1965;91:371–375. [DOI] [PubMed] [Google Scholar]
- 15.Lefer AM, Glenn TM. Role of the pancreas in the pathogenesis of circulatory shock. Adv Exp Med Biol. 1971;23:311–335. [DOI] [PubMed] [Google Scholar]
- 16.Glenn TM, Lefer AM. Significance of splanchnic proteases in the production of a toxic factor in hemorrhagic shock. Circ Res. 1971;29:338–349. [DOI] [PubMed] [Google Scholar]
- 17.Lefer AM, Spath JA Jr. Pancreatic hypoperfusion and the production of a myocardial depressant factor in hemorrhagic shock. Ann Surg. 1974;179:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manabe T, Suzuki T, Honjo I. Role of the pancreas in organ blood flow during shock. Surg Gynecol Obstet. 1978;146:577–82. [PubMed] [Google Scholar]
- 19.Kistler EB, Hugli TE, Schmid-Schonbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183–192. [PubMed] [Google Scholar]
- 20.Kistler EB, Lefer AM, Hugli TE, et al. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30–43. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. [DOI] [PubMed] [Google Scholar]
- 22.Deitch EA, Bridges W, Baker J, et al. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery. 1988;104:191–198. [PubMed] [Google Scholar]
- 23.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416. [PubMed] [Google Scholar]
- 24.Faries PL, Simon RJ, Martella AT, et al. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma. 1998;44:1031–1036. [DOI] [PubMed] [Google Scholar]
- 25.Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. [DOI] [PubMed] [Google Scholar]
- 26.Kanwar S, Windsor AC, Welsh F, et al. Lack of correlation between failure of gut barrier function and septic complications after major upper gastrointestinal surgery. Ann Surg. 2000;231:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roumen RM, van der Vliet JA, Weavers RA, et al. Intestinal permeability is increased after major vascular surgery. J Vasc Surg. 1993;17:734–737. [DOI] [PubMed] [Google Scholar]
- 28.Guo W, Ding J, Huang Q, et al. Alterations in the bacterial flora modulate the systemic cytokine response to hemorrhagic shock. Am J Physiol. 1995;269:G827–832. [DOI] [PubMed] [Google Scholar]
- 29.Haglund U, Rasmussen I. Oxygenation of the gut mucosa. Br J Surg. 1993;80:955–956. [DOI] [PubMed] [Google Scholar]
- 30.Zaets SB, Berezina TL, Caruso J, et al. Mesenteric lymph duct ligation prevents shock-induced RBC deformability and shape changes. J Surg Res. 2003. In press. [DOI] [PubMed]


