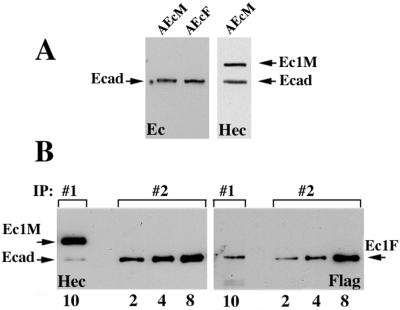
FIG. 3.
Relative amounts of the adhesive and lateral dimers in A-431 cells. (A) Confluent AEcM and AEcF cells were extracted with IP buffer, adjusted to the same protein concentration, and analyzed by immunoblotting with two different anti-E-cadherin antibodies, C20820 (Ec) or HECD-1 (Hec). Note that the amounts of endogenous E-cadherin (Ecad) in both cell lines were equal; the amounts of Ec1M (Ec1M) and endogenous cadherin in AEcM cells were also equal. (B) Ten microliters of the myc immunoprecipitate (IP:#1) obtained from AEcM/AEcF coculture and 2, 4, and 8 μl of the anti-E-cadherin (monoclonal antibody C20820) immunoprecipitate (IP:#2) obtained from a homogeneous AEcF culture were analyzed with HECD-1 (Hec) and anti-Flag (Flag) antibodies (the number of microliters of immunoprecipitate is shown below the blots). In IP#1, Ec1F was derived exclusively from adhesive dimers, while in IP#2, it was derived from both lateral and adhesive dimers. The staining with the HECD-1 antibody shows that 10 μl of IP#1 and 8 μl of IP#2 contain equal amounts of immunoprecipitated components, Ec1M (Ec1M) in IP#1 and E-cadherin (E-cad) in IP#2. Staining the identical blot with anti-Flag antibody shows that the amount of coimmunoprecipitated Ec1F in 10 μl of IP#1 is about four times less than in 8 μl of IP#2.